The rate of sputum smear-positive tuberculosis after treatment default in a high-burden setting: a retrospective cohort study
- PMID: 23049846
- PMCID: PMC3458061
- DOI: 10.1371/journal.pone.0045724
The rate of sputum smear-positive tuberculosis after treatment default in a high-burden setting: a retrospective cohort study
Erratum in
- PLoS One. 2013;8(8). doi: 10.1371/annotation/ea5f2a13-4394-41af-84c8-3e6af4a07770
Abstract
Rationale: High rates of recurrent tuberculosis after successful treatment have been reported from different high burden settings in Sub-Saharan Africa. However, little is known about the rate of smear-positive tuberculosis after treatment default. In particular, it is not known whether or not treatment defaulters continue to be or become again smear-positive and thus pose a potential for transmission of infection to others.
Objective: To investigate, in a high tuberculosis burden setting, the rate of re-treatment for smear-positive tuberculosis among cases defaulting from standardized treatment compared to successfully treated cases.
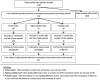
Methods: Retrospective cohort study among smear-positive tuberculosis cases treated between 1996 and 2008 in two urban communities in Cape Town, South Africa. Episodes of re-treatment for smear-positive tuberculosis were ascertained via probabilistic record linkage. Survival analysis and Poisson regression were used to compare the rate of smear-positive tuberculosis after treatment default to that after successful treatment.
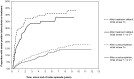
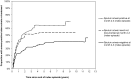
Results: A total of 2,136 smear-positive tuberculosis cases were included in the study. After treatment default, the rate of re-treatment for smear-positive tuberculosis was 6.86 (95% confidence interval [CI]: 5.59-8.41) per 100 person-years compared to 2.09 (95% CI: 1.81-2.41) after cure (adjusted Hazard Ratio [aHR]: 3.97; 95% CI: 3.00-5.26). Among defaulters, the rate was inversely associated with treatment duration and sputum conversion prior to defaulting. Smear grade at start of the index treatment episode (Smear3+: aHR 1.61; 95%CI 1.11-2.33) was independently associated with smear-positive tuberculosis re-treatment, regardless of treatment outcome.
Conclusions: In this high-burden setting, there is a high rate of subsequent smear-positive tuberculosis after treatment default. Treatment defaulters are therefore likely to contribute to the pool of infectious source cases in the community. Our findings underscore the importance of preventing treatment default, as a means of successful tuberculosis control in high-burden settings.
Conflict of interest statement
Figures

References
-
- Santha T (2004) What is the optimum duration of treatment? In: Toman’s Tuberculosis: Case Detection, Treatment, and Monitoring (p144); Geneva: World Health Organization, 2004. (ISBN: 9241546034).
-
- [No authors listed] (1981) Clinical trial of six-month and four-month regimens of chemotherapy in the treatment of pulmonary tuberculosis: the results up to 30 months. Tubercle 62: 95–102. - PubMed
-
- [No authors listed] (1986) Long-term follow-up of a clinical trial of six-month and four-month regimens of chemotherapy in the treatment of pulmonary tuberculosis. Singapore Tuberculosis Service/British Medical Research Council. Am Rev Respir Dis 133: 779–783. - PubMed
-
- [No authors listed] (1986) A controlled clinical trial of 3- and 5-month regimens in the treatment of sputum-positive pulmonary tuberculosis in South India. Tuberculosis Research Centre, Madras, and National Tuberculosis Institute, Bangalore. Am Rev Respir Dis 134: 27–33. - PubMed
-
- Dye C, Garnett GP, Sleeman K, Williams BG (1998) Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Directly observed short-course therapy. Lancet 352: 1886–1891. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

