Respiratory pathogens adopt a chronic lifestyle in response to bile
- PMID: 23049911
- PMCID: PMC3458808
- DOI: 10.1371/journal.pone.0045978
Respiratory pathogens adopt a chronic lifestyle in response to bile
Abstract
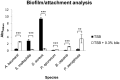
Chronic respiratory infections are a major cause of morbidity and mortality, most particularly in Cystic Fibrosis (CF) patients. The recent finding that gastro-esophageal reflux (GER) frequently occurs in CF patients led us to investigate the impact of bile on the behaviour of Pseudomonas aeruginosa and other CF-associated respiratory pathogens. Bile increased biofilm formation, Type Six Secretion, and quorum sensing in P. aeruginosa, all of which are associated with the switch from acute to persistent infection. Furthermore, bile negatively influenced Type Three Secretion and swarming motility in P. aeruginosa, phenotypes associated with acute infection. Bile also modulated biofilm formation in a range of other CF-associated respiratory pathogens, including Burkholderia cepacia and Staphylococcus aureus. Therefore, our results suggest that GER-derived bile may be a host determinant contributing to chronic respiratory infection.
Conflict of interest statement
Figures



Similar articles
-
Bile effects on the Pseudomonas aeruginosa pathogenesis in cystic fibrosis patients with gastroesophageal reflux.Heliyon. 2023 Nov 10;9(11):e22111. doi: 10.1016/j.heliyon.2023.e22111. eCollection 2023 Nov. Heliyon. 2023. PMID: 38034726 Free PMC article. Review.
-
The cep quorum-sensing system of Burkholderia cepacia H111 controls biofilm formation and swarming motility.Microbiology (Reading). 2001 Sep;147(Pt 9):2517-2528. doi: 10.1099/00221287-147-9-2517. Microbiology (Reading). 2001. PMID: 11535791
-
Direct detection of N-acylhomoserine lactones in cystic fibrosis sputum.FEMS Microbiol Lett. 2002 Jan 22;207(1):1-7. doi: 10.1111/j.1574-6968.2002.tb11019.x. FEMS Microbiol Lett. 2002. PMID: 11886742
-
Staphylococcus aureus Biofilm Growth on Cystic Fibrosis Airway Epithelial Cells Is Enhanced during Respiratory Syncytial Virus Coinfection.mSphere. 2018 Aug 15;3(4):e00341-18. doi: 10.1128/mSphere.00341-18. mSphere. 2018. PMID: 30111629 Free PMC article.
-
Pulmonary infections in patients with cystic fibrosis.Semin Respir Infect. 2002 Mar;17(1):47-56. doi: 10.1053/srin.2002.31690. Semin Respir Infect. 2002. PMID: 11891518 Review.
Cited by
-
Beyond pancreatic insufficiency and liver disease in cystic fibrosis.Eur J Pediatr. 2016 Jul;175(7):881-94. doi: 10.1007/s00431-016-2719-5. Epub 2016 Apr 7. Eur J Pediatr. 2016. PMID: 27055450 Review.
-
Systems Biology and Bile Acid Signalling in Microbiome-Host Interactions in the Cystic Fibrosis Lung.Antibiotics (Basel). 2021 Jun 24;10(7):766. doi: 10.3390/antibiotics10070766. Antibiotics (Basel). 2021. PMID: 34202495 Free PMC article. Review.
-
The Detection of Bile Acids in the Lungs of Paediatric Cystic Fibrosis Patients Is Associated with Altered Inflammatory Patterns.Diagnostics (Basel). 2020 May 6;10(5):282. doi: 10.3390/diagnostics10050282. Diagnostics (Basel). 2020. PMID: 32384684 Free PMC article.
-
Gastric Aspiration and Its Role in Airway Inflammation.Open Respir Med J. 2018 Jan 23;12:1-10. doi: 10.2174/1874306401812010001. eCollection 2018. Open Respir Med J. 2018. PMID: 29456774 Free PMC article. Review.
-
Bile acids repress hypoxia-inducible factor 1 signaling and modulate the airway immune response.Infect Immun. 2014 Sep;82(9):3531-41. doi: 10.1128/IAI.00674-13. Epub 2014 Jun 9. Infect Immun. 2014. PMID: 24914220 Free PMC article.
References
-
- D'Ovidio F, Singer LG, Hadjiliadis D, Pierre A, Waddell TK, et al. (2005) Prevalence of gastroesophageal reflux in end-stage lung disease candidates for lung transplant. Ann Thorac Surg 80: 1254–1260. - PubMed
-
- Pauwels A, Decraene A, Blondeau K, Mertens V, Farre R, et al... (2011) Bile Acids in Sputum and Increased Airway Inflammation in Patients with Cystic Fibrosis. Chest. - PubMed
-
- Perng DW, Chang KT, Su KC, Wu YC, Wu MT, et al. (2007) Exposure of airway epithelium to bile acids associated with gastroesophageal reflux symptoms: a relation to transforming growth factor-beta1 production and fibroblast proliferation. Chest 132: 1548–1556. - PubMed
-
- Perng DW, Wu YC, Tsai CC, Su KC, Liu LY, et al. (2008) Bile acids induce CCN2 production through p38 MAP kinase activation in human bronchial epithelial cells: a factor contributing to airway fibrosis. Respirology 13: 983–989. - PubMed
-
- Scott M, Gelhot AR (1999) Gastroesophageal reflux disease: diagnosis and management. Am Fam Physician 59: 1161–1169, 1199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

