Inhibition of doxorubicin-induced senescence by PPARδ activation agonists in cardiac muscle cells: cooperation between PPARδ and Bcl6
- PMID: 23049957
- PMCID: PMC3458009
- DOI: 10.1371/journal.pone.0046126
Inhibition of doxorubicin-induced senescence by PPARδ activation agonists in cardiac muscle cells: cooperation between PPARδ and Bcl6
Abstract
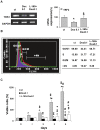
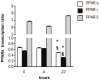
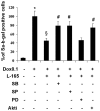
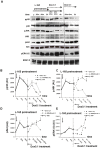
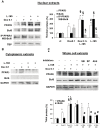
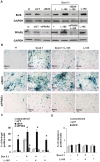
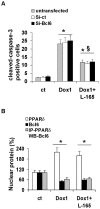
Senescence and apoptosis are two distinct cellular programs that are activated in response to a variety of stresses. Low or high doses of the same stressor, i.e., the anticancer drug doxorubicin, may either induce apoptosis or senescence, respectively, in cardiac muscle cells. We have demonstrated that PPARδ, a ligand-activated transcriptional factor that controls lipid metabolism, insulin sensitivity and inflammation, is also involved in the doxorubicin-induced senescence program. This occurs through its interference with the transcriptional repressor protein B cell lymphoma-6 (Bcl6). Low doses of doxorubicin increase the expression of PPARδ that sequesters Bcl6, thus preventing it from exerting its anti-senescent effects. We also found that L-165041, a specific PPARδ activator, is highly effective in protecting cardiomyocytes from doxorubicin-induced senescence through a Bcl6 related mechanism. In fact, L-165041 increases Bcl6 expression via p38, JNK and Akt activation, and at the same time it induces the release of Bcl6 from PPARδ, thereby enabling Bcl6 to bind to its target genes. L-165041 also prevented apoptosis induced by higher doses of doxorubicin. However, while experiments performed with siRNA analysis techniques very clearly showed the weight of Bcl6 in the cellular senescence program, no role was found for Bcl6 in the anti-apoptotic effects of L-165041, thus confirming that senescence and apoptosis are two very distinct stress response cellular programs. This study increases our understanding of the molecular mechanism of anthracycline cardiotoxicity and suggests a potential role for PPARδ agonists as cardioprotective agents.
Conflict of interest statement
Figures

References
-
- Hershman DL, McBride RB, Eisenberger A, Tsai WY, Grann VR, et al. (2008) Doxorubicin, cardiac risk factors, and cardiac toxicity in elderly patients with diffuse B-cell non-Hodgkin’s lymphoma. J Clin Oncol 26: 3159–3165. - PubMed
-
- Auner HW, Tinchon C, Linkesch W, Halwachs-Baumann G, Sill H (2001) Correspondence re: O. J. Arola, et al., (2000) Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis Cancer Res 60: 1789–1792. Cancer Res 61: 2335–2336. - PubMed
-
- Maejima Y, Adachi S, Ito H, Hirao K, Isobe M (2008) Induction of premature senescence in cardiomyocytes by doxorubicin as a novel mechanism of myocardial damage. Aging Cell 7: 125–136. - PubMed
-
- Spallarossa P, Altieri P, Aloi C, Garibaldi S, Barisione C, et al. (2009) Doxorubicin induces senescence or apoptosis in rat neonatal cardiomyocytes by regulating the expression levels of the telomere binding factors 1 and 2. Am J Physiol Heart Circ Physiol 297: H2169–H2181. - PubMed
-
- Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25: 585–621. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

