The EWS/FLI Oncogene Drives Changes in Cellular Morphology, Adhesion, and Migration in Ewing Sarcoma
- PMID: 23050043
- PMCID: PMC3463921
- DOI: 10.1177/1947601912457024
The EWS/FLI Oncogene Drives Changes in Cellular Morphology, Adhesion, and Migration in Ewing Sarcoma
Abstract
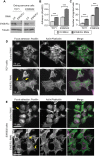
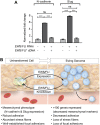
Ewing sarcoma is a tumor of the bone and soft tissue caused by the expression of a translocation-derived oncogenic transcription factor, EWS/FLI. Overt metastases are associated with a poor prognosis in Ewing sarcoma, but patients without overt metastases frequently harbor micrometastatic disease at presentation. This suggests that the metastatic potential of Ewing sarcoma exists at an early stage during tumor development. We have therefore explored whether the inciting oncogenic event in Ewing sarcoma, EWS/FLI, directly modulates tumor cell features that support metastasis, such as cell adhesion, cell migration, and cytoarchitecture. We used an RNAi-based approach in patient-derived Ewing sarcoma cell lines. Although we hypothesized that EWS/FLI might induce classic metastatic features, such as increased cell adhesion, migration, and invasion (similar to the phenotypes observed when epithelial malignancies undergo an epithelial-to-mesenchymal transition during the process of metastasis), surprisingly, we found the opposite. Thus, EWS/FLI expression inhibited the adhesion of isolated cells in culture and prevented adhesion in an in vivo mouse lung assay. Cell migration was similarly inhibited by EWS/FLI expression. Furthermore, EWS/FLI expression caused a striking loss of organized actin stress fibers and focal adhesions and a concomitant loss of cell spreading, suggesting that EWS/FLI disrupts the mesenchymal phenotype of a putative tumor cell-of-origin. These data suggest a new paradigm for the dissemination and metastasis of mesenchymally derived tumors: these tumors may disseminate via a "passive/stochastic" model rather than via an "active" epithelial-to-mesenchymal type transition. In the case of Ewing sarcoma, it appears that the loss of cell adhesion needed to promote tumor cell dissemination might be induced by the EWS/FLI oncogene itself rather than via an accumulation of stepwise mutations.
Keywords: EWS/FLI; Ewing sarcoma; cell adhesion; cytoskeleton; metastasis.
Conflict of interest statement
Figures

References
-
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559-64 - PubMed
-
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274-84 - PubMed
-
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14: 818-29 - PubMed
-
- Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402-5 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
