The inhibitory effect of S-nitrosoglutathione on blood-brain barrier disruption and peroxynitrite formation in a rat model of experimental stroke
- PMID: 23050646
- PMCID: PMC3481195
- DOI: 10.1111/j.1471-4159.2012.07947.x
The inhibitory effect of S-nitrosoglutathione on blood-brain barrier disruption and peroxynitrite formation in a rat model of experimental stroke
Abstract
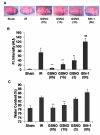
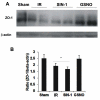
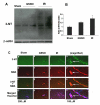
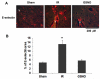
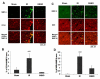
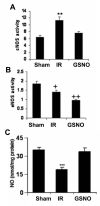
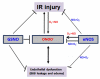
The hallmark of stroke injury is endothelial dysfunction leading to blood-brain barrier (BBB) leakage and edema. Among the causative factors of BBB disruption are accelerating peroxynitrite formation and the resultant decreased bioavailability of nitric oxide (NO). S-nitrosoglutathione (GSNO), an S-nitrosylating agent, was found not only to reduce the levels of peroxynitrite but also to protect the integrity of BBB in a rat model of cerebral ischemia and reperfusion (IR). A treatment with GSNO (3 μmol/kg) after IR reduced 3-nitrotyrosine levels in and around vessels and maintained NO levels in brain. This mechanism protected endothelial function by reducing BBB leakage, increasing the expression of Zonula occludens-1 (ZO-1), decreasing edema, and reducing the expression of matrix metalloproteinase-9 and E-selectin in the neurovascular unit. An administration of the peroxynitrite-forming agent 3-morpholino sydnonimine (3 μmol/kg) at reperfusion increased BBB leakage and decreased the expression of ZO-1, supporting the involvement of peroxynitrite in BBB disruption and edema. Mechanistically, the endothelium-protecting action of GSNO was invoked by reducing the activity of nuclear factor kappa B and increasing the expression of S-nitrosylated proteins. Taken together, the results support the ability of GSNO to improve endothelial function by reducing nitroxidative stress in stroke.
© 2012 The Authors Journal of Neurochemistry © International Society for Neurochemistry.
Figures


References
-
- Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. - PubMed
-
- Berti R, Williams AJ, Moffett JR, Hale SL, Velarde LC, Elliott PJ, Yao C, Dave JR, Tortella FC. Quantitative real-time RT-PCR analysis of inflammatory gene expression associated with ischemia-reperfusion brain injury. J Cereb Blood Flow Metab. 2002;22:1068–1079. - PubMed
-
- Chen HY, Chen TY, Lee MY, Chen ST, Hsu YS, Kuo YL, Chang GL, Wu TS, Lee EJ. Melatonin decreases neurovascular oxidative/nitrosative damage and protects against early increases in the blood-brain barrier permeability after transient focal cerebral ischemia in mice. J Pineal Res. 2006;41:175–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

