Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity
- PMID: 23050834
- PMCID: PMC3549212
- DOI: 10.1089/ars.2012.4623
Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity
Abstract
Aims: The sources of cytosolic superoxide in skeletal muscle have not been defined. This study examined the subcellular sites that contribute to cytosolic superoxide in mature single muscle fibers at rest and during contractile activity.
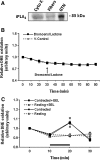
Results: Isolated fibers from mouse flexor digitorum brevis loaded with superoxide and nitric-oxide-sensitive fluorescent probes, specific pathway inhibitors and immunolocalization techniques were used to identify subcellular sites contributing to cytosolic superoxide. Treatment with the electron transport chain complex III inhibitor, antimycin A, but not the complex I inhibitor, rotenone, caused increased cytosolic superoxide through release from the mitochondrial intermembrane space via voltage-dependent anion or Bax channels, but inhibition of these channels did not affect contraction-induced increases in cytosolic superoxide. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitors decreased cytosolic superoxide at rest and following contractions. Protein and mRNA expression of NADPH oxidase subunits was demonstrated in single fibers. NOX2, NOX4, and p22(phox) subunits localized to the sarcolemma and transverse tubules; NOX4 was additionally expressed in mitochondria. Regulatory p40(phox) and p67(phox) proteins were found in the cytoplasm of resting fibers, but following contractions, p40(phox) appeared to translocate to the sarcolemma.
Innovation: Superoxide and other reactive oxygen species generated by skeletal muscle are important regulators of muscle force production and adaptations to contractions. This study has defined the relative contribution of mitochondrial and cytosolic sources of superoxide within the cytosol of single muscle fibers at rest and during contractions.
Conclusion: Muscle mitochondria do not modulate cytosolic superoxide in skeletal muscle but NADPH oxidase is a major contributor both at rest and during contractions.
Figures









References
-
- Abou-Sleiman PM. Muqit MM. Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat Rev Neurosci. 2006;7:207–219. - PubMed
-
- Ackermann EJ. Conde-Frieboes K. Dennis EA. Inhibition of macrophage Ca(2+)-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J Biol Chem. 1995;270:445–450. - PubMed
-
- Aydin J. Andersson DC. Hanninen SL. Wredenberg A. Tavi P. Park CB. Larsson NG. Bruton JD. Westerblad H. Increased mitochondrial Ca2+ and decreased sarcoplasmic reticulum Ca2+ in mitochondrial myopathy. Hum Mol Genet. 2009;18:278–288. - PubMed
-
- Balcerczyk A. Soszynski M. Rybaczek D. Przygodzki T. Karowicz-Bilinska A. Maszewski J. Bartosz G. Induction of apoptosis and modulation of production of reactive oxygen species in human endothelial cells by diphenyleneiodonium. Biochem Pharmacol. 2005;69:1263–1273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

