Molecular mechanisms of spatial protein quality control
- PMID: 23051707
- PMCID: PMC3510865
- DOI: 10.4161/pri.22470
Molecular mechanisms of spatial protein quality control
Abstract
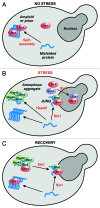
Evidence is now accumulating that damaged proteins are not randomly distributed but often concentrated in microscopically visible and functionally distinct inclusion bodies. How misfolded proteins are organized into these compartments, however, is still unknown. We have recently begun to investigate stress-inducible protein quality control (PQC) bodies in yeast cells. Surprisingly, we found that protein misfolding and aggregation were not sufficient to trigger body formation under mild heat stress conditions. Rather, compartment assembly also required the concerted action of molecular chaperones, protein-sorting factors and protein-sequestration factors, thus defining a minimal machinery for spatial PQC. Expression of this machinery was limited to times of acute stress through rapid changes in mRNA abundance and a proteasomal feedback mechanism. These findings demonstrate that yeast cells can control the amount of soluble misfolded proteins through regulated phase transitions in the cytoplasm, thus allowing them to rapidly adapt to changing environmental conditions.
Figures
Comment in
- Malinovska L, Kroschwald S, Munder MC, Richter D, Alberti S. Molecular chaperones and stress-inducible protein-sorting factors coordinate the spatiotemporal distribution of protein aggregates. Mol Biol Cell. 2012;23:3041–56. doi: 10.1091/mbc.E12-03-0194. doi: 10.1091/mbc.E12-03-0194
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
