Structure of the agonist-bound neurotensin receptor
- PMID: 23051748
- PMCID: PMC3482300
- DOI: 10.1038/nature11558
Structure of the agonist-bound neurotensin receptor
Abstract
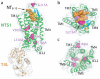
Neurotensin (NTS) is a 13-amino-acid peptide that functions as both a neurotransmitter and a hormone through the activation of the neurotensin receptor NTSR1, a G-protein-coupled receptor (GPCR). In the brain, NTS modulates the activity of dopaminergic systems, opioid-independent analgesia, and the inhibition of food intake; in the gut, NTS regulates a range of digestive processes. Here we present the structure at 2.8 Å resolution of Rattus norvegicus NTSR1 in an active-like state, bound to NTS(8-13), the carboxy-terminal portion of NTS responsible for agonist-induced activation of the receptor. The peptide agonist binds to NTSR1 in an extended conformation nearly perpendicular to the membrane plane, with the C terminus oriented towards the receptor core. Our findings provide, to our knowledge, the first insight into the binding mode of a peptide agonist to a GPCR and may support the development of non-peptide ligands that could be useful in the treatment of neurological disorders, cancer and obesity.
Figures




Comment in
-
Structural biology: Snapshot of an activated peptide receptor.Nature. 2012 Oct 25;490(7421):492-3. doi: 10.1038/nature11634. Epub 2012 Oct 10. Nature. 2012. PMID: 23051750 No abstract available.
-
G protein-coupled receptors: Pioneering peptide GPCR structure determined.Nat Rev Drug Discov. 2012 Nov;11(11):832. doi: 10.1038/nrd3875. Nat Rev Drug Discov. 2012. PMID: 23123937 No abstract available.
References
-
- Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J. Biol. Chem. 1973;248:6854–6861. - PubMed
-
- Bissette G, Nemeroff CB, Loosen PT, Prange AJ, Jr., Lipton MA. Hypothermia and intolerance to cold induced by intracisternal administration of the hypothalamic peptide neurotensin. Nature. 1976;262:607–609. - PubMed
-
- Carraway RE, Plona AM. Involvement of neurotensin in cancer growth: evidence, mechanisms and development of diagnostic tools. Peptides. 2006;27:2445–2460. - PubMed
-
- Griebel G, Holsboer F. Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat. Rev. Drug Discov. 2012;11:462–478. - PubMed
-
- Kitabgi P. Targeting neurotensin receptors with agonists and antagonists for therapeutic purposes. Curr. Opin. Drug Discov. Devel. 2002;5:764–776. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

