Quantitative 3-dimensional corneal imaging in vivo using a modified HRT-RCM confocal microscope
- PMID: 23051907
- PMCID: PMC3546130
- DOI: 10.1097/ICO.0b013e31825ec44e
Quantitative 3-dimensional corneal imaging in vivo using a modified HRT-RCM confocal microscope
Abstract
Purpose: The purpose of this study was to develop and test hardware and software modifications to allow quantitative full-thickness corneal imaging using the Heidelberg Retina Tomograph (HRT) Rostock Corneal Module.
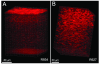
Methods: A personal computer-controlled motor drive with positional feedback was integrated into the system to allow automated focusing through the entire cornea. The left eyes of 10 New Zealand white rabbits were scanned from endothelium to epithelium. Image sequences were read into a custom-developed program for depth calculation and measurement of sublayer thicknesses. Three-dimensional visualizations were also generated using Imaris. In 6 rabbits, stack images were registered, and depth-dependent counts of keratocyte nuclei were made using Metamorph.
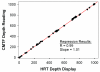
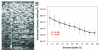
Results: The mean epithelial and corneal thickness measured in the rabbit were 47 ± 5 μm and 373 ± 25 μm, respectively (n = 10 corneas); coefficients of variation for repeated scans were 8.2% and 2.1%. Corneal thickness measured using ultrasonic pachymetry was 374 + 17 μm. The mean overall keratocyte density measured in the rabbit was 43,246 ± 5603 cells per cubic millimeter in vivo (n = 6 corneas). There was a gradual decrease in keratocyte density from the anterior to posterior cornea (R = 0.99), consistent with previous data generated in vitro.
Conclusion: This modified system allows high-resolution 3-dimensional image stacks to be collected from the full-thickness rabbit cornea in vivo. These data sets can be used for interactive visualization of corneal cell layers, measurement of sublayer thickness, and depth-dependent keratocyte density measurements. Overall, the modifications significantly expand the potential quantitative research applications of the HRT Rostock Cornea Module microscope.
Figures
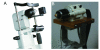


References
-
- Efron N. Contact lens-induced changes in the anterior eye as observed in vivo with the confocal microscope. Prog Retin Eye Res. 2007;26:398–436. - PubMed
-
- Dhaliwal JS, Kaufman SC, Chiou AGY. Current applications of clinical confocal microscopy. Curr Opin Ophthalmol. 2007;18:300–307. - PubMed
-
- Labbe A, Khammari C, Dupas B, et al. Contribution of in vivo confocal microscopy to the diagnosis and management of infectious keratitis. The Ocular Surface. 2009;7:41–52. - PubMed
-
- Petran M, Hadravsky M, Egger MD, Galambos R. Tandem scanning reflected light microscope. J Opt Soc Am. 1968;58:661–664.
-
- Petran M, Hadravsky M, Benes J, Kucera R, Boyde A. The tandem scanning reflected light microscope: Part I: the principle, and its design. Proc R Microsc Soc. 1985;20:125–129.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

