Prostate specific antigen testing policy worldwide varies greatly and seems not to be in accordance with guidelines: a systematic review
- PMID: 23052017
- PMCID: PMC3528621
- DOI: 10.1186/1471-2296-13-100
Prostate specific antigen testing policy worldwide varies greatly and seems not to be in accordance with guidelines: a systematic review
Abstract
Background: Prostate specific antigen (PSA) testing is widely used, but guidelines on follow-up are unclear.
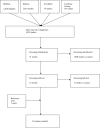
Methods: We performed a systematic review of the literature to determine follow-up policy after PSA testing by general practitioners (GPs) and non-urologic hospitalists, the use of a cut-off value for this policy, the reasons for repeating a PSA test after an initial normal result, the existence of a general cut-off value below which a PSA result is considered normal, and the time frame for repeating a test. Data sources. MEDLINE, Embase, PsychInfo and the Cochrane library from January 1950 until May 2011. Study eligibility criteria. Studies describing follow-up policy by GPs or non-urologic hospitalists after a primary PSA test, excluding urologists and patients with prostate cancer. Studies written in Dutch, English, French, German, Italian or Spanish were included. Excluded were studies describing follow-up policy by urologists and follow-up of patients with prostate cancer. The quality of each study was structurally assessed.
Results: Fifteen articles met the inclusion criteria. Three studies were of high quality. Follow-up differed greatly both after a normal and an abnormal PSA test result. Only one study described the reasons for not performing follow-up after an abnormal PSA result.
Conclusions: Based on the available literature, we cannot adequately assess physicians' follow-up policy after a primary PSA test. Follow-up after a normal or raised PSA test by GPs and non-urologic hospitalists seems to a large extent not in accordance with the guidelines.
Comment in
-
Re: Prostate specific antigen testing policy worldwide varies greatly and seems not to be in accordance with guidelines: a systematic review.J Urol. 2013 Nov;190(5):1766. doi: 10.1016/j.juro.2013.07.078. Epub 2013 Aug 2. J Urol. 2013. PMID: 24120783 No abstract available.
References
-
- Vedel I, Puts MTE, Monette M, Monette J, Bergman H. The decision-making process in prostate cancer screening in primary care with a prostate-specific antigen: A systematic review. J Geriatr Oncol. 2011;2011 http://dx.doi.org/10.1016/j.jgo.2011.04.001. - DOI
-
- Djulbegovic M, Beyth RJ, Neuberger MM, Stoffs TL, Vieweg J, Djulbegovic B, Dahm P. Screening for prostate cancer: systematic review and meta-analysis of randomised controlled trials. BMJ. 2010;2010 http://dx.doi.org/10.1136/bmj.c4543. - DOI - PMC - PubMed
-
- Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Määttänen L, Bangma CH, Aus G, Villers A, Rebillard X, Van der Kwast T, Blijenberg BG, Moss SM, De Koning HJ, Auvinen A. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous