Reelin-Disabled-1 signaling in neuronal migration: splicing takes the stage
- PMID: 23052211
- PMCID: PMC4457513
- DOI: 10.1007/s00018-012-1171-6
Reelin-Disabled-1 signaling in neuronal migration: splicing takes the stage
Abstract
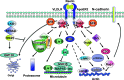
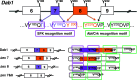
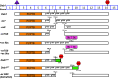
Reelin-Disabled-1 (Dab1) signaling has a well-established role in regulating neuronal migration during brain development. Binding of Reelin to its receptors induces Dab1 tyrosine phosphorylation. Tyrosine-phosphorylated Dab1 recruits a wide range of SH2 domain-containing proteins and activates multiple signaling cascades, resulting in cytoskeleton remodeling and precise neuronal positioning. In this review, we summarize recent progress in the Reelin-Dab1 signaling field. We focus on Dab1 alternative splicing as a mechanism for modulating the Reelin signal in developing brain. We suggest that correct positioning of neurons in the developing brain is at least partly controlled by alternatively-spliced Dab1 isoforms that differ in the number and type of tyrosine phosphorylation motifs that they contain. We propose a model whereby different subsets of SH2 domain-containing proteins are activated by different Dab1 isoforms, resulting in coordinated migration of neurons.
Figures


Similar articles
-
Splice-mediated motif switching regulates disabled-1 phosphorylation and SH2 domain interactions.Mol Cell Biol. 2012 Jul;32(14):2794-808. doi: 10.1128/MCB.00570-12. Epub 2012 May 14. Mol Cell Biol. 2012. PMID: 22586277 Free PMC article.
-
Relative importance of the tyrosine phosphorylation sites of Disabled-1 to the transmission of Reelin signaling.Brain Res. 2009 Dec 22;1304:26-37. doi: 10.1016/j.brainres.2009.09.087. Epub 2009 Sep 29. Brain Res. 2009. PMID: 19796633
-
Migration of sympathetic preganglionic neurons in the spinal cord is regulated by Reelin-dependent Dab1 tyrosine phosphorylation and CrkL.J Comp Neurol. 2007 Jun 1;502(4):635-43. doi: 10.1002/cne.21318. J Comp Neurol. 2007. PMID: 17394141
-
Nonneuronal roles for the reelin signaling pathway.Dev Dyn. 2017 Apr;246(4):217-226. doi: 10.1002/dvdy.24462. Epub 2016 Nov 17. Dev Dyn. 2017. PMID: 27739126 Review.
-
The functions of Reelin in membrane trafficking and cytoskeletal dynamics: implications for neuronal migration, polarization and differentiation.Biochem J. 2017 Sep 7;474(18):3137-3165. doi: 10.1042/BCJ20160628. Biochem J. 2017. PMID: 28887403 Review.
Cited by
-
Alternative Splicing in Neurogenesis and Brain Development.Front Mol Biosci. 2018 Feb 12;5:12. doi: 10.3389/fmolb.2018.00012. eCollection 2018. Front Mol Biosci. 2018. PMID: 29484299 Free PMC article. Review.
-
QTL and systems genetics analysis of mouse grooming and behavioral responses to novelty in an open field.Genes Brain Behav. 2017 Nov;16(8):790-799. doi: 10.1111/gbb.12392. Epub 2017 Jun 22. Genes Brain Behav. 2017. PMID: 28544613 Free PMC article.
-
Downregulation of DAB2IP promotes mesenchymal-to-neuroepithelial transition and neuronal differentiation of human mesenchymal stem cells.PLoS One. 2013 Sep 20;8(9):e75884. doi: 10.1371/journal.pone.0075884. eCollection 2013. PLoS One. 2013. PMID: 24073285 Free PMC article.
-
Immunoexpression Pattern of Autophagy Markers in Developing and Postnatal Kidneys of Dab1-/-(yotari) Mice.Biomolecules. 2023 Feb 21;13(3):402. doi: 10.3390/biom13030402. Biomolecules. 2023. PMID: 36979337 Free PMC article.
-
Whole-genome analysis reveals the contribution of non-coding de novo transposon insertions to autism spectrum disorder.Mob DNA. 2021 Nov 27;12(1):28. doi: 10.1186/s13100-021-00256-w. Mob DNA. 2021. PMID: 34838103 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

