Neural stem cell engraftment and myelination in the human brain
- PMID: 23052294
- PMCID: PMC3893824
- DOI: 10.1126/scitranslmed.3004373
Neural stem cell engraftment and myelination in the human brain
Erratum in
- Sci Transl Med. 2012 Dec 19;4(165):165er8
Abstract
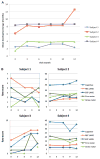
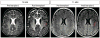
Pelizaeus-Merzbacher disease (PMD) is a rare leukodystrophy caused by mutation of the proteolipid protein 1 gene. Defective oligodendrocytes in PMD fail to myelinate axons, causing global neurological dysfunction. Human central nervous system stem cells (HuCNS-SCs) can develop into oligodendrocytes and confer structurally normal myelin when transplanted into a hypomyelinating mouse model. A 1-year, open-label phase-1 study was undertaken to evaluate safety and to detect evidence of myelin formation after HuCNS-SC transplantation. Allogeneic HuCNS-SCs were surgically implanted into the frontal lobe white matter in four male subjects with an early-onset severe form of PMD. Immunosuppression was administered for 9 months. Serial neurological evaluations, developmental assessments, and cranial magnetic resonance imaging (MRI) and MR spectroscopy, including high-angular resolution diffusion tensor imaging (DTI), were performed at baseline and after transplantation. The neurosurgical procedure, immunosuppression regimen, and HuCNS-SC transplantation were well tolerated. Modest gains in neurological function were observed in three of the four subjects. No clinical or radiological adverse effects were directly attributed to the donor cells. Reduced T1 and T2 relaxation times were observed in the regions of transplantation 9 months after the procedure in the three subjects. Normalized DTI showed increasing fractional anisotropy and reduced radial diffusivity, consistent with myelination, in the region of transplantation compared to control white matter regions remote to the transplant sites. These phase 1 findings indicate a favorable safety profile for HuCNS-SCs in subjects with PMD. The MRI results suggest durable cell engraftment and donor-derived myelin in the transplanted host white matter.
Conflict of interest statement
Figures



Comment in
-
White matter disease: myelination achieved by transplanted neural stem cells.Nat Rev Neurol. 2012 Dec;8(12):659. doi: 10.1038/nrneurol.2012.224. Epub 2012 Nov 13. Nat Rev Neurol. 2012. PMID: 23147855 No abstract available.
References
-
- Schneider A, Montague P, Griffiths I, Fanarraga M, Kennedy P, Brophy P, Nave KA. Uncoupling of hypomyelination and glial cell death by a mutation in the proteolipid protein gene. Nature. 1992;358:758–761. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

