ISG56/IFIT1 is primarily responsible for interferon-induced changes to patterns of parainfluenza virus type 5 transcription and protein synthesis
- PMID: 23052390
- PMCID: PMC3542720
- DOI: 10.1099/vir.0.046797-0
ISG56/IFIT1 is primarily responsible for interferon-induced changes to patterns of parainfluenza virus type 5 transcription and protein synthesis
Abstract
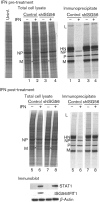
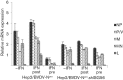
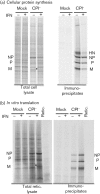
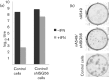
Interferon (IFN) induces an antiviral state in cells that results in alterations of the patterns and levels of parainfluenza virus type 5 (PIV5) transcripts and proteins. This study reports that IFN-stimulated gene 56/IFN-induced protein with tetratricopeptide repeats 1 (ISG56/IFIT1) is primarily responsible for these effects of IFN. It was shown that treating cells with IFN after infection resulted in an increase in virus transcription but an overall decrease in virus protein synthesis. As there was no obvious decrease in the overall levels of cellular protein synthesis in infected cells treated with IFN, these results suggested that ISG56/IFIT1 selectively inhibits the translation of viral mRNAs. This conclusion was supported by in vitro translation studies. Previous work has shown that ISG56/IFIT1 can restrict the replication of viruses lacking a 2'-O-methyltransferase activity, an enzyme that methylates the 2'-hydroxyl group of ribose sugars in the 5'-cap structures of mRNA. However, the data in the current study strongly suggested that PIV5 mRNAs are methylated at the 2'-hydroxyl group and thus that ISG56/IFIT1 selectively inhibits the translation of PIV5 mRNA by some as yet unrecognized mechanism. It was also shown that ISG56/IFIT1 is primarily responsible for the IFN-induced inhibition of PIV5.
Figures




References
-
- Andrejeva J., Childs K. S., Young D. F., Carlos T. S., Stock N., Goodbourn S., Randall R. E. (2004). The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-β promoter. Proc Natl Acad Sci U S A 101, 17264–17269 10.1073/pnas.0407639101 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

