Development of the serotonergic cells in murine raphe nuclei and their relations with rhombomeric domains
- PMID: 23052546
- PMCID: PMC3748323
- DOI: 10.1007/s00429-012-0456-8
Development of the serotonergic cells in murine raphe nuclei and their relations with rhombomeric domains
Abstract
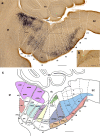
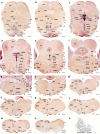
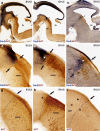
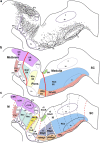
The raphe nuclei represent the origin of central serotonergic projections. The literature distinguishes seven nuclei grouped into rostral and caudal clusters relative to the pons. The boundaries of these nuclei have not been defined precisely enough, particularly with regard to developmental units, notably hindbrain rhombomeres. We hold that a developmental point of view considering rhombomeres may explain observed differences in connectivity and function. There are twelve rhombomeres characterized by particular genetic profiles, and each develops between one and four distinct serotonergic populations. We have studied the distribution of the conventional seven raphe nuclei among these twelve units. To this aim, we correlated 5-HT-immunoreacted neurons with rhombomeric boundary landmarks in sagittal mouse brain sections at different developmental stages. Furthermore, we performed a partial genoarchitectonic analysis of the developing raphe nuclei, mapping all known serotonergic differentiation markers, and compared these results, jointly with others found in the literature, with our map of serotonin-containing populations, in order to examine regional variations in correspondence. Examples of regionally selective gene patterns were identified. As a result, we produced a rhombomeric classification of some 45 serotonergic populations, and suggested a corresponding modified terminology. Only a minor rostral part of the dorsal raphe nucleus lies in the midbrain. Some serotonergic neurons were found in rhombomere 4, contrary to the conventional assumption that it lacks such neurons. We expect that our reclassification of raphe nuclei may be useful for causal analysis of their differential molecular specification, as well as for studies of differential connectivity and function.
Figures











References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

