Effects of formulation on the bioavailability of lutein and zeaxanthin: a randomized, double-blind, cross-over, comparative, single-dose study in healthy subjects
- PMID: 23052623
- PMCID: PMC3663991
- DOI: 10.1007/s00394-012-0447-9
Effects of formulation on the bioavailability of lutein and zeaxanthin: a randomized, double-blind, cross-over, comparative, single-dose study in healthy subjects
Abstract
Purpose: Lutein and zeaxanthin are macular pigments with a protective function in the retina. These xanthophylls must be obtained from the diet or added to foods or supplements via easy-to-use, stable formulations. The technique employed to produce these formulations may affect the bioavailability of the xanthophylls.
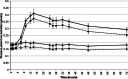
Methods: Forty-eight healthy volunteers were randomized into this double-blind, cross-over study investigating the plasma kinetics of lutein provided as two different beadlet formulations. Subjects (n = 48) received a single dose of 20 mg of lutein as either a starch-matrix ("SMB", FloraGLO® Lutein 5 %) or as a cross-linked alginate-matrix beadlet ("AMB", Lyc-O-Lutein 20 %) formulation. Plasma concentrations of lutein and zeaxanthin were measured at 0, 1, 3, 6, 9, 12, 14, 24, 26, 28, 32, 36, 48, 72, 168, and 672 h.
Results: The mean plasma AUC(0-72h), AUC(0-672h), and C(max) for total lutein and zeaxanthin and their all-E-isomers were significantly increased (p < 0.001) from pre-dose concentrations in response to SMB and AMB. There was no difference in lutein T max between the two test articles. However, by 14 h post-dose, total plasma lutein increased by 7 % with AMB and by 126 % with SMB. Total lutein AUC(0-72h) and AUC(0-672h) were 1.8-fold and 1.3-fold higher, respectively, for SMB compared to AMB. Both formulations were well tolerated by subjects in this study.
Conclusion: These findings confirm that the bioavailability of lutein and zeaxanthin critically depends on the formulation used and document a superiority of the starch-based over the alginate-based product in this study.
Figures




References
-
- Burton GW. Antioxidant action of carotenoids. J Nutr. 1989;119:109–111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

