The toxicity outcome of silica nanoparticles (Ludox®) is influenced by testing techniques and treatment modalities
- PMID: 23053168
- PMCID: PMC3462312
- DOI: 10.1007/s00216-012-6246-6
The toxicity outcome of silica nanoparticles (Ludox®) is influenced by testing techniques and treatment modalities
Abstract
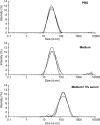
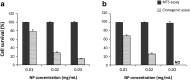
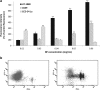
We analyzed the influence of the kind of cytotoxicity test and its application modality in defining the level of hazard of the in vitro exposures to nanostructures. We assessed the cytotoxicity induced by two different Ludox® silica nanoparticles (NPs), AS30 and SM30, on three human cell lines, CCD-34Lu, A549, and HT-1080. Dynamic light scattering measurements showed particle agglomeration when NPs are diluted in culture medium supplemented with fetal calf serum. We examined the impact of such particle aggregation on the cytotoxicity by exposing the cells to NPs under different treatment modalities: short incubation (2 h) in serum-free medium or long incubation (24-72 h) in serum-containing medium. Under this last modality, NP suspensions tended to form aggregates and were toxic at concentrations five- to tenfold higher than in serum-free medium. The results of cell survival varied considerably when the long-term clonogenic assay was performed to validate the data of the short-term MTS assay. Indeed, the half maximum effective concentrations (EC(50)) in all the three cell lines were four- to fivefold lower when calculated from the data of clonogenic assay than of MTS. Moreover, the mechanisms of NP toxicity were cell-type-specific, showing that CCD-34Lu are prone to the induction of plasma membrane damages and HT-1080 are prone to DNA double-strand break and apoptosis induction. Taken together, our results demonstrate that the choice of testing strategy and treatment conditions plays an important role in assessing the in vitro toxicity of NPs.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

