Development of a lipopolysaccharide-targeted peptide mimic vaccine against Q fever
- PMID: 23053512
- PMCID: PMC3833726
- DOI: 10.4049/jimmunol.1201622
Development of a lipopolysaccharide-targeted peptide mimic vaccine against Q fever
Abstract
Coxiella burnetii is a Gram-negative bacterium that causes acute and chronic Q fever in humans. Creation of a safe and effective new generation vaccine to prevent Q fever remains an important public health goal. Previous studies suggested that Ab-mediated immunity to C. burnetii phase I LPS (PI-LPS) is protective. To identify the potential peptides that can mimic the protective epitopes on PI-LPS, a PI-LPS-specific mAb 1E4 was generated, characterized, and used to screen a phage display library. Interestingly, our results indicate that 1E4 was able to inhibit C. burnetii infection in vivo, suggesting that 1E4 is a protective mAb. After three rounds of biopanning by 1E4 from the phage display library, a mimetic peptide, m1E41920, was identified, chemically synthesized, and conjugated to keyhole limpet hemocyanin (KLH) for examining its immunogenicity. The results indicate that the synthetic peptide m1E41920 was able to inhibit the binding of 1E4 to PI Ag, suggesting m1E41920 shares the same binding site of 1E4 with the epitopes of PI Ag. In addition, m1E41920-KLH elicited a specific IgG response to PI Ag, and immune sera from m1E41920-KLH-immunized mice was able to inhibit C. burnetii infection in vivo, suggesting that m1E41920 may specifically mimic the protective epitope of PI-LPS. Furthermore, m1E41920-KLH was able to confer significant protection against C. burnetii challenge. Thus, m1E41920-KLH is a protective Ag and may be useful for developing a safe and effective vaccine against Q fever. This study demonstrates the feasibility of developing a peptide mimic vaccine against Q fever.
Figures
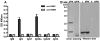

Similar articles
-
Characterization of a lipopolysaccharide-targeted monoclonal antibody and its variable fragments as candidates for prophylaxis against the obligate intracellular bacterial pathogen Coxiella burnetii.Infect Immun. 2014 Nov;82(11):4530-41. doi: 10.1128/IAI.01695-14. Epub 2014 Aug 11. Infect Immun. 2014. PMID: 25114119 Free PMC article.
-
Mechanisms of vaccine-induced protective immunity against Coxiella burnetii infection in BALB/c mice.J Immunol. 2007 Dec 15;179(12):8372-80. doi: 10.4049/jimmunol.179.12.8372. J Immunol. 2007. PMID: 18056383
-
Chemokine Receptor 7 Is Essential for Coxiella burnetii Whole-Cell Vaccine-Induced Cellular Immunity but Dispensable for Vaccine-Mediated Protective Immunity.J Infect Dis. 2019 Jul 19;220(4):624-634. doi: 10.1093/infdis/jiz146. J Infect Dis. 2019. PMID: 30938819 Free PMC article.
-
Components of protective immunity.Adv Exp Med Biol. 2012;984:91-104. doi: 10.1007/978-94-007-4315-1_5. Adv Exp Med Biol. 2012. PMID: 22711628 Review.
-
Vaccines against coxiellosis and Q fever. Development of a chloroform:methanol residue subunit of phase I Coxiella burnetti for the immunization of animals.Ann N Y Acad Sci. 1992 Jun 16;653:88-111. doi: 10.1111/j.1749-6632.1992.tb19633.x. Ann N Y Acad Sci. 1992. PMID: 1626897 Review.
Cited by
-
Putative phage-display epitopes of the porcine epidemic diarrhea virus S1 protein and their anti-viral activity.Virus Genes. 2015 Oct;51(2):217-24. doi: 10.1007/s11262-015-1234-5. Epub 2015 Aug 21. Virus Genes. 2015. PMID: 26292945 Free PMC article.
-
Advancement and applications of peptide phage display technology in biomedical science.J Biomed Sci. 2016 Jan 19;23:8. doi: 10.1186/s12929-016-0223-x. J Biomed Sci. 2016. PMID: 26786672 Free PMC article.
-
Genetic mechanisms of Coxiella burnetii lipopolysaccharide phase variation.PLoS Pathog. 2018 Feb 26;14(3):e1006922. doi: 10.1371/journal.ppat.1006922. eCollection 2018 Mar. PLoS Pathog. 2018. PMID: 29481553 Free PMC article.
-
Coxiella burnetii: international pathogen of mystery.Microbes Infect. 2020 Apr;22(3):100-110. doi: 10.1016/j.micinf.2019.09.001. Epub 2019 Sep 28. Microbes Infect. 2020. PMID: 31574310 Free PMC article. Review.
-
Characterization of a lipopolysaccharide-targeted monoclonal antibody and its variable fragments as candidates for prophylaxis against the obligate intracellular bacterial pathogen Coxiella burnetii.Infect Immun. 2014 Nov;82(11):4530-41. doi: 10.1128/IAI.01695-14. Epub 2014 Aug 11. Infect Immun. 2014. PMID: 25114119 Free PMC article.
References
-
- Angelakis E, Raoult D. Q Fever. Vet Microbiol. 2010;140:297–309. - PubMed
-
- Bell JF, Lackman DB, Meis A, Hadlow WJ. Recurrent Reaction of Site of Q Fever Vaccination in a Sensitized Person. Mil Med. 1964;129:591–5. - PubMed
-
- Marmion BP, Ormsbee RA, Kyrkou M, et al. Vaccine prophylaxis of abattoir-associated Q fever. Lancet. 1984;2:1411–4. - PubMed
-
- Ackland JR, Worswick DA, Marmion BP. Vaccine prophylaxis of Q fever. A follow-up study of the efficacy of Q-Vax (CSL) 1985–1990. Med J Aust. 1994;160:704–8. - PubMed
-
- Stoker MG, Fiset P. Phase variation of the Nine Mile and other strains of Rickettsia burnetii. Can J Microbiol. 1956;2:310–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

