In vivo knockdown of intersectin-1s alters endothelial cell phenotype and causes microvascular remodeling in the mouse lungs
- PMID: 23054079
- PMCID: PMC3543613
- DOI: 10.1007/s10495-012-0762-x
In vivo knockdown of intersectin-1s alters endothelial cell phenotype and causes microvascular remodeling in the mouse lungs
Abstract
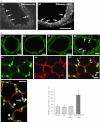
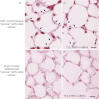
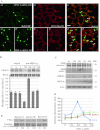
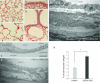
Intersectin-1s (ITSN-1s) is a general endocytic protein involved in regulating lung vascular permeability and endothelial cells (ECs) survival, via MEK/Erk1/2(MAPK) signaling. To investigate the in vivo effects of ITSN-1s deficiency and the resulting ECs apoptosis on pulmonary vasculature and lung homeostasis, we used an ITSN-1s knocked-down (KD(ITSN)) mouse generated by repeated delivery of a specific siRNA targeting ITSN-1 gene (siRNA(ITSN)). Biochemical and histological analyses as well as electron microscopy (EM) revealed that acute KD(ITSN) [3-days (3d) post-siRNA(ITSN) treatment] inhibited Erk1/2(MAPK) pro-survival signaling, causing significant ECs apoptosis and lung injury; at 10d of KD(ITSN), caspase-3 activation was at peak, terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)-positive ECs showed 3.4-fold increase, the mean linear intercept (MLI) showed 48 % augment and pulmonary microvessel density as revealed by aquaporin-1 staining (AQP-1) decreased by 30 %, all compared to controls; pulmonary function was altered. Concomitantly, expression of several growth factors known to activate Erk1/2(MAPK) and suppress Bad pro-apoptotic activity increased. KD(ITSN) altered Smads activity, downstream of the transforming growth factor beta-receptor-1 (TβR1), as shown by subcellular fractionation and immunoblot analyses. Moreover, 24d post-siRNA(ITSN), surviving ECs became hyper-proliferative and apoptotic-resistant against ITSN-1s deficiency, as demonstrated by EM imaging, 5-bromo-deoxyuridine (BrdU) incorporation and Bad-Ser(112/155) phosphorylation, respectively, leading to increased microvessel density and repair of the injured lungs, as well as matrix deposition. In sum, ECs endocytic dysfunction and apoptotic death caused by KD(ITSN) contribute to the initial lung injury and microvascular loss, followed by endothelial phenotypic changes and microvascular remodeling in the remaining murine pulmonary microvascular bed.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

