Microglial activation and antioxidant responses induced by the Parkinson's disease protein α-synuclein
- PMID: 23054368
- PMCID: PMC3582877
- DOI: 10.1007/s11481-012-9401-0
Microglial activation and antioxidant responses induced by the Parkinson's disease protein α-synuclein
Abstract
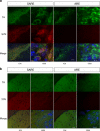
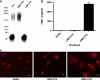
Parkinson's disease (PD) is the second most common age-related neurodegenerative disorder typified by tremor, rigidity, akinesia and postural instability due in part to the loss of dopamine within the nigrostriatal system. The pathologic features of this disorder include the loss of substantia nigra dopamine neurons and attendant striatal terminals, the presence of large protein-rich neuronal inclusions containing fibrillar α-synuclein and increased numbers of activated microglia. Evidence suggests that both misfolded α-synuclein and oxidative stress play an important role in the pathogenesis of sporadic PD. Here we review evidence that α-synuclein activates glia inducing inflammation and that Nrf2-directed phase-II antioxidant enzymes play an important role in PD. We also provide new evidence that the expression of antioxidant enzymes regulated in part by Nrf2 is increased in a mouse model of α-synuclein overexpression. We show that misfolded α-synuclein directly activates microglia inducing the production and release of the proinflammatory cytokine, TNF-α, and increasing antioxidant enzyme expression. Importantly, we demonstrate that the precise structure of α-synuclein is important for induction of this proinflammatory pathway. This complex α-synuclein-directed glial response highlights the importance of protein misfolding, oxidative stress and inflammation in PD and represents a potential locus for the development of novel therapeutics focused on induction of the Nrf2-directed antioxidant pathway and inhibition of protein misfolding.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures