Inhibiting interactions of lysine demethylase LSD1 with snail/slug blocks cancer cell invasion
- PMID: 23054398
- PMCID: PMC3537890
- DOI: 10.1158/0008-5472.CAN-12-1739
Inhibiting interactions of lysine demethylase LSD1 with snail/slug blocks cancer cell invasion
Abstract
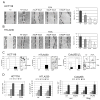
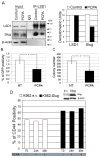
The process of epithelial-mesenchymal transition (EMT) which is required for cancer cell invasion is regulated by a family of E-box-binding transcription repressors, which include Snail (SNAIL1) and Slug (SNAI2). Snail appears to repress the expression of the EMT marker E-cadherin by epigenetic mechanisms dependent on the interaction of its N-terminal SNAG domain with chromatin-modifying proteins including lysine-specific demethylase 1 (LSD1/KDM1A). We assessed whether blocking Snail/Slug-LSD1 interaction by treatment with Parnate, an enzymatic inhibitor of LSD1, or TAT-SNAG, a cell-permeable peptide corresponding to the SNAG domain of Slug, suppresses the motility and invasiveness of cancer cells of different origin and genetic background. We show here that either treatment blocked Slug-dependent repression of the E-cadherin promoter and inhibited the motility and invasion of tumor cell lines without any effect on their proliferation. These effects correlated with induction of epithelial and repression of mesenchymal markers and were phenocopied by LSD1 or Slug downregulation. Parnate treatment also inhibited bone marrow homing/engraftment of Slug-expressing K562 cells. Together, these studies support the concept that targeting Snail/Slug-dependent transcription repression complexes may lead to the development of novel drugs selectively inhibiting the invasive potential of cancer cells.
Conflict of interest statement
Figures




References
-
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Medicine. 2006;12:895–904. - PubMed
-
- Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. - PubMed
-
- Martin-Belmonte F, Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer. 2012;12:23–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

