3-year real-world outcomes with the Swedish adjustable gastric band™ in France
- PMID: 23054572
- PMCID: PMC3560940
- DOI: 10.1007/s11695-012-0765-2
3-year real-world outcomes with the Swedish adjustable gastric band™ in France
Abstract
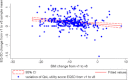
The study objective was to ascertain outcomes with the Swedish adjustable gastric band (SAGB) on an intention-to-treat basis in multiple centers across the French social health insurance system. SAGB results at 3-year follow-up are reported. The noncomparative, observational, prospective, consecutive cohort study design sought a 500-patient minimum recruitment geographically representative of continental France. Safety (adverse events [AEs], device-related morbidity, and mortality) and effectiveness (change in body mass index [BMI, kilograms per square meter], percentage excess weight loss, comorbidities, quality of life [QoL]) were assessed. Adjustable gastric band survival was calculated. Thirty-one surgeons in 28 multidisciplinary teams/sites enrolled patients between September 2, 2007 and April 30, 2008. SAGB was successfully implanted in 517 patients: 88.0 % female; mean age, 37.5 years; obesity duration, 15.3 years (baseline: mean BMI, 41.0; comorbidities, 773 in 74.3 % of patients; Bariatric Analysis and Reporting Outcome System (BAROS), 1.4; EuroQoL 5-Dimensions (EQ-5D), 0.61; EuroQoL-visual analog scale (EQ-VAS), 52.3). At 3 years: BMI, 32.2 (mean change, -9.0; p < 0.0001); excess weight loss, 47.4 %; comorbidities, 161 in 27.2 %; BAROS, 3.6 (+2.2, p < 0.0001); EQ-5D, 0.84 (+0.22, p < 0.0001); EQ-VAS, 73.4 (+21.4, p < 0.0001). SAGB-induced weight loss was associated with substantially improved QoL. One death occurred and was unrelated to the treatment. No AE was reported in 68.3 % of patients, and no confirmed device-related AE in 77.0 %. Overall AE rate was 0.19 per patient year. Device retention was 87.0 %. Analysis of patients lost to follow-up showed a nonsignificant effect on overall study results. In a prospective, consecutive cohort, "real-world", nationwide study, the Swedish Adjustable Gastric Band was found safe and effective at 3-year follow-up.
Figures




References
-
- Avis de la CEPP du 1er septembre 2004 modifié le 20 octobre 2004 SAGB (anneau gastrique ajustable suédois), implant annulaire ajustable pour gastroplastie. Modèles 2100-X et 2200-X. http://www.santedev.org/biblio/pdf2/has/cepp/2004/10/20/pp020367.pdf. Accessed: March 12, 2012
-
- Avis de la CEPP 8 mars 2006 SAGB (anneau gastrique ajustable suédois) Quick-Close fourni avec le site VELOCITY et son applicateur, implant annulaire ajustable pour gastroplastie. Modèle BD2XV.
-
- Association Française d’Etudes et de Recherches sur l’Obésité, Association de Langue Française pour l’Etude du Diabète et des Maladies Métaboliques, Société de Nutrition et de Diététique de Langue Française Recommandations pour le diagnostic, la prévention et le traitement des obésités en France. Cah Nutr Diet. 1998;33:10–42.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

