Daclizumab therapy for multiple sclerosis
- PMID: 23055048
- PMCID: PMC3557356
- DOI: 10.1007/s13311-012-0147-4
Daclizumab therapy for multiple sclerosis
Abstract
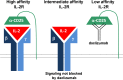
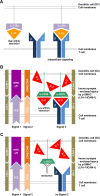
Daclizumab is a humanized monoclonal antibody of IgG1 subtype that binds to the Tac epitope on the interleukin-2 (IL-2) receptor α-chain (CD25), thus, effectively blocking the formation of the high-affinity IL-2 receptor. Because the high-affinity IL-2 receptor signaling promotes expansion of activated T cells in vitro, daclizumab was designed as a therapy that selectively inhibits T-cell activation. Assuming the previous statement, daclizumab received regulatory approval as add-on therapy to standard immunosuppressive regimen for the prevention of acute allograft rejection in renal transplantation. Based on its putative mechanism of action (MOA), daclizumab represented an ideal therapy for T-cell-mediated autoimmune diseases and was subsequently tested in inflammatory uveitis and multiple sclerosis (MS). In both of these diseases, daclizumab therapy significantly inhibited target organ inflammation. Mechanistic studies in MS demonstrated that the MOA of daclizumab is surprisingly broad and that the drug exerts unexpected effects on multiple components of the innate immune system. Specifically, daclizumab dramatically expands and activates immunoregulatory CD56(bright) NK cells, which gain access to the intrathecal compartment in MS and can kill autologous activated T cells. Daclizumab also blocks trans-presentation of IL-2 by mature dendritic cells to primed T cells, resulting in profound inhibition of antigen-specific T cells. Finally, daclizumab modulates the development of innate lymphoid cells. In conclusion, daclizumab therapy, which is currently in phase III testing for inflammatory MS, has a unique MOA that does not limit migration of immune cells into the intrathecal compartment, but rather provides multifactorial immunomodulatory effects with resultant inhibition of MS-related inflammation.
Figures
References
-
- Ruscetti FW, Morgan DA, Gallo RC. Functional and morphologic characterization of human T cells continuously grown in vitro. J Immunol. 1977;119:131–138. - PubMed
-
- Waldmann TA, Kozak RW, Tsudo M, Oh-ishi T, Bongiovanni KF, Goldman CK. IL-2 receptors in adult T-cell leukemia: a target for immunotherapy. Hamatol Bluttransfus. 1987;31:110–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

