Cambrian bivalved arthropod reveals origin of arthrodization
- PMID: 23055069
- PMCID: PMC3497099
- DOI: 10.1098/rspb.2012.1958
Cambrian bivalved arthropod reveals origin of arthrodization
Abstract
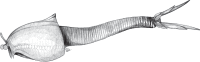
Extant arthropods are diverse and ubiquitous, forming a major constituent of most modern ecosystems. Evidence from early Palaeozoic Konservat Lagerstätten indicates that this has been the case since the Cambrian. Despite this, the details of arthropod origins remain obscure, although most hypotheses regard the first arthropods as benthic predators or scavengers such as the fuxianhuiids or megacheirans ('great-appendage' arthropods). Here, we describe a new arthropod from the Tulip Beds locality of the Burgess Shale Formation (Cambrian, series 3, stage 5) that possesses a weakly sclerotized thorax with filamentous appendages, encased in a bivalved carapace, and a strongly sclerotized, elongate abdomen and telson. A cladistic analysis resolved this taxon as the basal-most member of a paraphyletic grade of nekto-benthic forms with bivalved carapaces. This grade occurs at the base of Arthropoda (panarthropods with arthropodized trunk limbs) and suggests that arthrodization (sclerotization and jointing of the exoskeleton) evolved to facilitate swimming. Predatory and fully benthic habits evolved later in the euarthropod stem-lineage and are plesiomorphically retained in pycnogonids (sea spiders) and euchelicerates (horseshoe crabs and arachnids).
Figures


References
-
- Edgecombe G. D. 2010. Arthropod phylogeny: an overview from the perspectives of morphology, molecular data and the fossil record. Arthropod Struct. Dev. 39, 74–8710.1016/j.asd.2009.10.002 (doi:10.1016/j.asd.2009.10.002) - DOI - DOI - PubMed
-
- Budd G. E., Telford M. J. 2009. The origin and evolution of arthropods. Nature 457, 812–81710.1038/nature07890 (doi:10.1038/nature07890) - DOI - DOI - PubMed
-
- Legg D. A., Ma X., Wolfe J. M., Ortega-Hernández J., Edgecombe G. D., Sutton M. D. 2011. Lobopodian phylogeny reanalysed. Nature 476, E2–E310.1038/nature10267 (doi:10.1038/nature10267) - DOI - DOI - PubMed
-
- Briggs D. E. G., Erwin D. H., Collier F. J. 1994. The fossils of the Burgess Shale. Washington, DC: Smithsonian Institution
-
- Hou X. G., Aldridge R. J., Bergström J., Siveter D. J., Siveter D. J., Feng X. H. 2004. The Cambrian fossils of Chengjiang, China: the flowering of early animal life. Oxford, UK: Blackwell
MeSH terms
LinkOut - more resources
Full Text Sources

