Mass spectrometry analysis reveals non-mutated apolipoprotein A1 lumbosacral radiculoplexus amyloidoma
- PMID: 23055319
- PMCID: PMC3471670
- DOI: 10.1002/mus.23415
Mass spectrometry analysis reveals non-mutated apolipoprotein A1 lumbosacral radiculoplexus amyloidoma
Abstract
Introduction: In rare instances, amyloidosis presents as a focal, macroscopic lesion involving peripheral neural tissues (amyloidoma). In all known reported cases, peripheral nerve amyloidomas have had immunoglobulin light-chain fibril composition and occurred in the context of paraproteinemia.
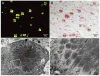
Methods: A 46-year-old man presented with progressive insidious-onset right lumbosacral radiculoplexus neuropathy without paraproteinemia. MRI-targeted fascicular nerve biopsy was performed on an enlarged sciatic nerve after earlier distal fibular nerve biopsy was nondiagnostic. Laser dissected mass spectroscopy of the discovered amyloid protein was performed after immunohistochemistry failed to identify the specific amyloid protein. Complete gene sequencing of apolipoprotein A1 (ApoA1) was performed.
Results: Only wild-type ApoA1 amyloid was found in the congophilic component in the nerve.
Conclusions: This case highlights the utility of MRI-guided fascicular nerve biopsy combined with laser-dissected mass spectrometric analysis. Importantly, the case expands the known causes of amyloidomas to include wild-type ApoA1.
Copyright © 2012 Wiley Periodicals, Inc.
Figures



Similar articles
-
Unusual amyloid polyneuropathy with predominant lumbosacral nerve roots and plexus involvement.Neurology. 1991 Feb;41(2 ( Pt 1)):206-8. doi: 10.1212/wnl.41.2_part_1.206. Neurology. 1991. PMID: 1846952
-
Amyloidogenicity and clinical phenotype associated with five novel mutations in apolipoprotein A-I.Am J Pathol. 2011 Oct;179(4):1978-87. doi: 10.1016/j.ajpath.2011.06.024. Epub 2011 Aug 5. Am J Pathol. 2011. PMID: 21820994 Free PMC article.
-
Two novel APOA1 gene mutations in a Japanese renal transplant recipient with recurrent apolipoprotein A-I related amyloidosis.Nephrology (Carlton). 2018 Jul;23 Suppl 2:17-21. doi: 10.1111/nep.13278. Nephrology (Carlton). 2018. PMID: 29968409
-
A new apolipoprotein Al variant, Trp50Arg, causes hereditary amyloidosis.QJM. 1995 Oct;88(10):695-702. QJM. 1995. PMID: 7493166 Review.
-
APOA1 related amyloidosis: a case report and literature review.Clin Biochem. 2003 Nov;36(8):641-5. doi: 10.1016/s0009-9120(03)00110-3. Clin Biochem. 2003. PMID: 14636880 Review.
Cited by
-
MRI of pathology-proven peripheral nerve amyloidosis.Skeletal Radiol. 2017 Jan;46(1):65-73. doi: 10.1007/s00256-016-2510-8. Epub 2016 Oct 12. Skeletal Radiol. 2017. PMID: 27730358
-
Apolipoprotein A-1-related amyloidosis 2 case reports and review of the literature.Medicine (Baltimore). 2017 Sep;96(39):e8148. doi: 10.1097/MD.0000000000008148. Medicine (Baltimore). 2017. PMID: 28953655 Free PMC article.
-
Myeloperoxidase-mediated Methionine Oxidation Promotes an Amyloidogenic Outcome for Apolipoprotein A-I.J Biol Chem. 2015 Apr 24;290(17):10958-71. doi: 10.1074/jbc.M114.630442. Epub 2015 Mar 10. J Biol Chem. 2015. PMID: 25759391 Free PMC article.
-
Methionine oxidized apolipoprotein A-I at the crossroads of HDL biogenesis and amyloid formation.FASEB J. 2018 Jun;32(6):3149-3165. doi: 10.1096/fj.201701127R. Epub 2018 Jan 17. FASEB J. 2018. PMID: 29401604 Free PMC article.
References
-
- Adams D. Hereditary and acquired amyloid neuropathies. J Neurol. 2001 Aug;248(8):647–657. - PubMed
-
- Kyle RA, Bayrd ED. Amyloidosis: review of 236 cases. Medicine (Baltimore) 1975 Jul;54(4):271–299. - PubMed
-
- Dyck PJ, Lambert EH. Dissociated sensation in amylidosis. Compound action potential, quantitative histologic and teased-fiber, and electron microscopic studies of sural nerve biopsies. Arch Neurol. 1969 May;20(5):490–507. - PubMed
-
- Kyle A, Kelly JJ, Dyck PJ. Amyloidosis and neuropathy. In: Dyck P, editor. Peripheral Neuropathies. Vol. 2. Philadelphia, PA: Saunders; 2005. pp. 2427–2451.
-
- Haridas A, Basu S, King A, Pollock J. Primary isolated amyloidoma of the lumbar spine causing neurological compromise: case report and literature review. Neurosurgery. 2005 Jul;57(1):E196. discussion E196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

