Aging affects neural precision of speech encoding
- PMID: 23055485
- PMCID: PMC3488287
- DOI: 10.1523/JNEUROSCI.2176-12.2012
Aging affects neural precision of speech encoding
Abstract
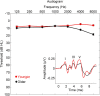
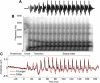
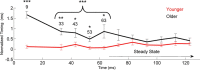
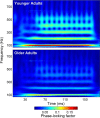
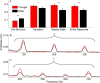
Older adults frequently report they can hear what is said but cannot understand the meaning, especially in noise. This difficulty may arise from the inability to process rapidly changing elements of speech. Aging is accompanied by a general slowing of neural processing and decreased neural inhibition, both of which likely interfere with temporal processing in auditory and other sensory domains. Age-related reductions in inhibitory neurotransmitter levels and delayed neural recovery can contribute to decreases in the temporal precision of the auditory system. Decreased precision may lead to neural timing delays, reductions in neural response magnitude, and a disadvantage in processing the rapid acoustic changes in speech. The auditory brainstem response (ABR), a scalp-recorded electrical potential, is known for its ability to capture precise neural synchrony within subcortical auditory nuclei; therefore, we hypothesized that a loss of temporal precision results in subcortical timing delays and decreases in response consistency and magnitude. To assess this hypothesis, we recorded ABRs to the speech syllable /da/ in normal hearing younger (18-30 years old) and older (60-67 years old) adult humans. Older adults had delayed ABRs, especially in response to the rapidly changing formant transition, and greater response variability. We also found that older adults had decreased phase locking and smaller response magnitudes than younger adults. Together, our results support the theory that older adults have a loss of temporal precision in the subcortical encoding of sound, which may account, at least in part, for their difficulties with speech perception.
Figures
References
-
- Aiken SJ, Picton TW. Envelope and spectral frequency-following responses to vowel sounds. Hear Res. 2008;245:35–47. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
