A new vesicular scaffolding complex mediates the G-protein-coupled 5-HT1A receptor targeting to neuronal dendrites
- PMID: 23055492
- PMCID: PMC6622363
- DOI: 10.1523/JNEUROSCI.6329-11.2012
A new vesicular scaffolding complex mediates the G-protein-coupled 5-HT1A receptor targeting to neuronal dendrites
Abstract
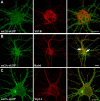
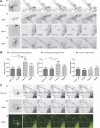
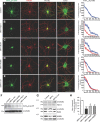
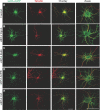
Although essential for their neuronal function, the molecular mechanisms underlying the dendritic targeting of serotonin G-protein-coupled receptors are poorly understood. Here, we characterized a Yif1B-dependent vesicular scaffolding complex mediating the intracellular traffic of the rat 5-HT(1A) receptor (5-HT(1A)R) toward dendrites. By combining directed mutagenesis, GST-pull down, and surface plasmon resonance, we identified a tribasic motif in the C-tail of the 5-HT(1A)R on which Yif1B binds directly with high affinity (K(D) ≈ 37 nM). Moreover, we identified Yip1A, Rab6, and Kif5B as new partners of the 5-HT(1A)R/Yif1B complex, and showed that their expression in neurons is also crucial for the dendritic targeting of the 5-HT(1A)R. Live videomicroscopy revealed that 5-HT(1A)R, Yif1B, Yip1A, and Rab6 traffic in vesicles exiting the soma toward the dendritic tree, and also exhibit bidirectional motions, sustaining their role in 5-HT(1A)R dendritic targeting. Hence, we propose a new trafficking pathway model in which Yif1B is the scaffold protein recruiting the 5-HT(1A)R in a complex including Yip1A and Rab6, with Kif5B and dynein as two opposite molecular motors coordinating the traffic of vesicles along dendritic microtubules. This targeting pathway opens new insights for G-protein-coupled receptors trafficking in neurons.
Conflict of interest statement
The authors declare no financial conflicts of interest.
Figures

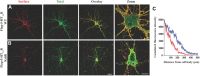
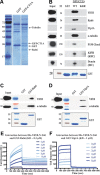
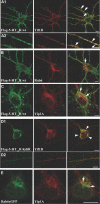
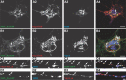
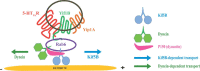
References
-
- Albert PR, Zhou QY, Van Tol HH, Bunzow JR, Civelli O. Cloning, functional expression, and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. J Biol Chem. 1990;265:5825–5832. - PubMed
-
- Barrowman J, Wang W, Zhang Y, Ferro-Novick S. The Yip1p.Yif1p complex is required for the fusion competence of endoplasmic reticulum-derived vesicles. J Biol Chem. 2003;278:19878–19884. - PubMed
-
- Björk K, Sjögren B, Svenningsson P. Regulation of serotonin receptor function in the nervous system by lipid rafts and adaptor proteins. Exp Cell Res. 2010;316:1351–1356. - PubMed
-
- Bockaert J, Perroy J, Bécamel C, Marin P, Fagni L. GPCR interacting proteins (GIPs) in the nervous system: roles in physiology and pathologies. Annu Rev Pharmacol Toxicol. 2010;50:89–109. - PubMed
-
- Bortolozzi A, Castañé A, Semakova J, Santana N, Alvarado G, Cortés R, Ferrés-Coy A, Fernández G, Carmona MC, Toth M, Perales JC, Montefeltro A, Artigas F. Selective siRNA-mediated suppression of 5-HT(1A) autoreceptors evokes strong anti-depressant-like effects. Mol Psychiatry. 2012;17:612–623. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
