Ectopic expression of select innexins in individual central neurons couples them to pre-existing neuronal or glial networks that express the same innexin
- PMID: 23055495
- PMCID: PMC3703444
- DOI: 10.1523/JNEUROSCI.2693-12.2012
Ectopic expression of select innexins in individual central neurons couples them to pre-existing neuronal or glial networks that express the same innexin
Abstract
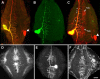
Fifteen of the 21 innexin (Inx) genes (Hve-inx) found in the genome of the medicinal leech, Hirudo verbana, are expressed in the CNS (Kandarian et al., 2012). Two are expressed pan-neuronally, while the others are restricted in their expression to small numbers of cells, in some cases reflecting the membership of known networks of electrically coupled and dye-coupled neurons or glial cells. We report here that when Hve-inx genes characteristic of discrete coupled networks were expressed ectopically in neurons known not to express them, the experimental cells were found to become dye coupled with the other cells in that network. Hve-inx6 is normally expressed by only three neurons in each ganglion, which form strongly dye-coupled electrical connections with each other [Shortening-Coupling interneuron (S-CI) network] (Muller and Scott, 1981; Dykes and Macagno, 2006). But when Hve-inx6 was ectopically expressed in a variety of central embryonic neurons, those cells became dye coupled with the S-CI network. Similarly, Hve-inx2 is normally uniquely expressed by the ganglion's large glial cells, but when it was ectopically expressed in different central neurons, they became dye coupled to the glial cells. In contrast, overexpression of the pan-neuronal Inx genes Hve-inx1 and Hve-inx14 did not yield any novel instances of dye coupling to pre-existent neuronal networks. These results reveal that expression of certain innexins is sufficient to couple individual neurons to pre-existing networks in the CNS. We propose that a primary determinant of selective neuronal connectivity and circuit formation in the leech is the surface expression of unique subsets of gap junctional proteins.
Figures


References
-
- Baccus SA, Burrell BD, Sahley CL, Muller KJ. Action potential reflection and failure at axon branch points cause stepwise changes in EPSPs in a neuron essential for learning. J Neurophysiol. 2000;83:1693–1700. - PubMed
-
- Baker MW, Macagno ER. Characterization of Hirudo medicinalis DNA promoters for targeted gene expression. J Neurosci Methods. 2006;156:145–153. - PubMed
-
- Chang M, Werner R, Dahl G. A role for an inhibitory connexin in testis? Dev Biol. 1996;175:50–56. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
