Identification of location and kinetically defined mechanism of cofactors and reporter genes in the cascade of steroid-regulated transactivation
- PMID: 23055525
- PMCID: PMC3510801
- DOI: 10.1074/jbc.M112.414805
Identification of location and kinetically defined mechanism of cofactors and reporter genes in the cascade of steroid-regulated transactivation
Abstract
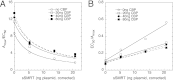
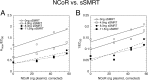
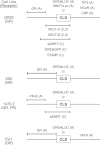
A currently obscure area of steroid hormone action is where the component factors, including receptor and reporter gene, act. The DNA binding of factors can be precisely defined, but the location and timing of factor binding and action are usually not equivalent. These questions are addressed for several factors (e.g. glucocorticoid receptor (GR), reporter, TIF2, NCoR, NELF-A, sSMRT, and STAMP) using our recently developed competition assay. This assay reveals both the kinetically defined mechanism of factor action and where the above factors act relative to both each other and the equilibrium equivalent to the rate-limiting step, which we call the concentration limiting step (CLS). The utility of this competition assay would be greatly increased if the position of the CLS is invariant and if the factor acting at the CLS is known. Here we report that the exogenous GREtkLUC reporter acts at the CLS as an accelerator for gene induction by GRs in U2OS cells. This mechanism of reporter function at the CLS persists with different reporters, factors, receptors, and cell types. We, therefore, propose that the reporter gene always acts at the CLS during gene induction and constitutes a landmark around which one can order the actions of all other factors. Current data suggest that how and where GR and the short form of SMRT act is also constant. These results validate a novel and rational methodology for identifying distally acting factors that would be attractive targets for pharmaceutical intervention in the treatment of diseases involving GR-regulated genes.
Figures



References
-
- Rosenfeld M. G., Glass C. K. (2001) Coregulator codes of transcriptional regulation by nuclear receptors. J. Biol. Chem. 276, 36865–36868 - PubMed
-
- Nagy L., Schwabe J. W. (2004) Mechanism of the nuclear receptor molecular switch. Trends Biochem. Sci. 29, 317–324 - PubMed
-
- Rosenfeld M. G., Lunyak V. V., Glass C. K. (2006) Sensors and signals. A coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 20, 1405–1428 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

