Lebrikizumab in the personalized management of asthma
- PMID: 23055690
- PMCID: PMC3459551
- DOI: 10.2147/BTT.S28666
Lebrikizumab in the personalized management of asthma
Abstract
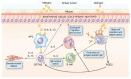
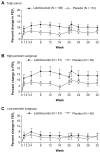
There is a need for improved therapies for severe asthma. Lebrikizumab, a humanized monoclonal antibody that binds to interleukin (IL)-13, is under development for the treatment of poorly controlled asthma. This article reviews the potential role of IL-13 in the pathogenesis of asthma, the efficacy and safety of lebrikizumab in humans, and progress in patient selection for lebrikizumab therapy. IL-13 is a T-helper (Th2) cell-derived cytokine implicated in inflammatory responses in asthma, including serum immunoglobulin-E synthesis, mucus hypersecretion, and subepithelial fibrosis. Blocking the pro-inflammatory effects of IL-13 with lebrikizumab has the potential to improve asthma control. Published data on the efficacy and safety of lebrikizumab in the treatment of asthma are relatively limited. The late asthmatic response after inhaled allergen challenge is reduced by almost 50%, following treatment with lebrikizumab. In a Phase II study performed in 219 adults with poorly controlled asthma despite inhaled corticosteroids (MILLY trial), lebrikizumab produced an improvement in prebronchodilator forced expiratory volume in 1 second of 5.5% compared with placebo at 12 weeks, but had no effects on other efficacy end points. Adverse effects were similar to placebo, except that musculoskeletal side effects occurred slightly more often with lebrikizumab. Stratifying patients into a high Th2 phenotype using serum periostin, which is upregulated in lung epithelial cells by IL-13, may identify individuals responsive to blockade of IL-13. In the MILLY trial, lebrikizumab treatment was associated with greater improvement in lung function in patients with elevated serum periostin levels compared with those with low periostin levels. Two large Phase III randomized controlled trials in patients with uncontrolled asthma are underway to establish the safety and efficacy of lebrikizumab when administered over a 52-week period. These studies will also help to determine whether identifying patients with a Th2 high inflammatory phenotype using serum periostin allows a personalized approach to the treatment of asthma.
Keywords: asthma; exhaled nitric oxide; interleukin-13; lebrikizumab; periostin; phenotypes.
Figures
References
-
- Robinson DS, Hamid Q, Ying S, et al. Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. - PubMed
-
- Holt PG, McCaubus C, Stumbles PA, Sly PD. The role of allergy in the development of asthma. Nature. 1999;402:B12–B17. - PubMed
-
- Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8(3):183–192. - PubMed
-
- Szefler S, Martin R, King T, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol. 2002;109(3):410–418. - PubMed
-
- Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved?: the gaining optimal asthma control study. Am J Respir Crit Care Med. 2004;170(8):836–844. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

