A Multi-Vector, Multi-Envelope HIV-1 Vaccine
- PMID: 23055844
- PMCID: PMC3462093
- DOI: 10.5863/1551-6776-12.2.68
A Multi-Vector, Multi-Envelope HIV-1 Vaccine
Abstract
The St. Jude Children's Research Hospital (St. Jude) HIV-1 vaccine program is based on the observation that multiple antigenically distinct HIV-1 envelope protein structures are capable of mediating HIV-1 infection. A cocktail vaccine comprising representatives of these diverse structures (immunotypes) is therefore considered necessary to elicit lymphocyte populations that prevent HIV-1 infection. This strategy is reminiscent of that used to design a currently licensed and successful 23-valent pneumococcus vaccine. Three recombinant vector systems are used for the delivery of envelope cocktails (DNA, vaccinia virus, and purified protein), and each of these has been tested individually in phase I safety trials. A fourth FDA-approved clinical trial, in which diverse envelopes and vectors are combined in a prime-boost vaccination regimen, has recently begun. This trial will continue to test the hypothesis that a multi-vector, multi-envelope vaccine can elicit diverse B- and T-cell populations that can prevent HIV-1 infections in humans.
Keywords: HIV-1 vaccine; Immunology; clinical trial; envelope; multi-vector.
Figures
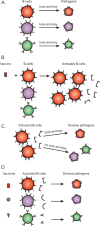
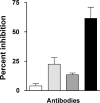
 ) B24 only. (
) B24 only. ( ) 114-9C10 only. (▪) B24 + 114-9C10. A combination of antibodies mediates synergistic virus neutralization. Mice were vaccinated with a single HIV-1IIIB–derived envelope or with a combination of envelopes including envelope from HIV-1IIIB. Delivery vehicles included recombinant DNA, vaccinia virus, and protein. Splenic cells from vaccinated mice were then harvested for B-cell hybridoma production. Once stable hybridomas (B24 and 114-9C10) were derived and cloned, respective antibodies were harvested from hybridoma culture media and purified by affinity chromatography with protein G sepharose. The neutralization assay was initiated by incubating HIV-1IIIB (approximately 10 TCID-50 per well in a 96-well microtiter plate) with or without antibodies in R10 medium (RPMI 1640 plus 10% heat-treated fetal bovine serum, penicillin, streptomycin and 4mM glutamine). Antibodies were used at concentrations selected to yield fractional virus inhibition, in order to measure additive or synergistic effects (final concentrations of B24 and 114-9C10 were 0.05 μg/mL and 10 μg/mL, respectively). After overnight incubation, the contents of wells were transferred to confluent GHOST-CXCR4 cells in 96-well plates and incubated overnight. Cells were washed with R10 medium and incubated an additional 3 days, after which supernatants were analyzed for virus growth with a Coulter HIV-1 p24 antigen assay (Beckman-Coulter, Miami, FL). Percent inhibition was determined by comparing test wells with wells containing virus and no antibody. Control wells were with virus and HIV-1 negative human serum.
) 114-9C10 only. (▪) B24 + 114-9C10. A combination of antibodies mediates synergistic virus neutralization. Mice were vaccinated with a single HIV-1IIIB–derived envelope or with a combination of envelopes including envelope from HIV-1IIIB. Delivery vehicles included recombinant DNA, vaccinia virus, and protein. Splenic cells from vaccinated mice were then harvested for B-cell hybridoma production. Once stable hybridomas (B24 and 114-9C10) were derived and cloned, respective antibodies were harvested from hybridoma culture media and purified by affinity chromatography with protein G sepharose. The neutralization assay was initiated by incubating HIV-1IIIB (approximately 10 TCID-50 per well in a 96-well microtiter plate) with or without antibodies in R10 medium (RPMI 1640 plus 10% heat-treated fetal bovine serum, penicillin, streptomycin and 4mM glutamine). Antibodies were used at concentrations selected to yield fractional virus inhibition, in order to measure additive or synergistic effects (final concentrations of B24 and 114-9C10 were 0.05 μg/mL and 10 μg/mL, respectively). After overnight incubation, the contents of wells were transferred to confluent GHOST-CXCR4 cells in 96-well plates and incubated overnight. Cells were washed with R10 medium and incubated an additional 3 days, after which supernatants were analyzed for virus growth with a Coulter HIV-1 p24 antigen assay (Beckman-Coulter, Miami, FL). Percent inhibition was determined by comparing test wells with wells containing virus and no antibody. Control wells were with virus and HIV-1 negative human serum.
Similar articles
-
UV-inactivated vaccinia virus (VV) in a multi-envelope DNA-VV-protein (DVP) HIV-1 vaccine protects macaques from lethal challenge with heterologous SHIV.Vaccine. 2012 May 2;30(21):3188-95. doi: 10.1016/j.vaccine.2012.03.001. Epub 2012 Mar 14. Vaccine. 2012. PMID: 22425790 Free PMC article.
-
Multi-envelope HIV vaccine safety and immunogenicity in small animals and chimpanzees.Immunol Res. 2000;21(1):7-21. doi: 10.1385/IR:21:1:7. Immunol Res. 2000. PMID: 10803879
-
HIV-1 vaccine development: tackling virus diversity with a multi-envelope cocktail.Front Biosci. 2008 Jan 1;13:609-20. doi: 10.2741/2706. Front Biosci. 2008. PMID: 17981574 Review.
-
Priming with a Potent HIV-1 DNA Vaccine Frames the Quality of Immune Responses prior to a Poxvirus and Protein Boost.J Virol. 2019 Jan 17;93(3):e01529-18. doi: 10.1128/JVI.01529-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30429343 Free PMC article.
-
Novel prime-boost vaccine strategies against HIV-1.Expert Rev Vaccines. 2019 Aug;18(8):765-779. doi: 10.1080/14760584.2019.1640117. Epub 2019 Jul 9. Expert Rev Vaccines. 2019. PMID: 31271322 Review.
Cited by
-
Multi-Envelope HIV-1 Vaccine Development: Two Targeted Immune Pathways, One Desired Protective Outcome.Viral Immunol. 2018 Mar;31(2):124-132. doi: 10.1089/vim.2017.0144. Epub 2018 Jan 9. Viral Immunol. 2018. PMID: 29315059 Free PMC article. Review.
-
Glucopyranosyl lipid A adjuvant significantly enhances HIV specific T and B cell responses elicited by a DNA-MVA-protein vaccine regimen.PLoS One. 2014 Jan 23;9(1):e84707. doi: 10.1371/journal.pone.0084707. eCollection 2014. PLoS One. 2014. PMID: 24465426 Free PMC article.
-
A Comparative Phase I Study of Combination, Homologous Subtype-C DNA, MVA, and Env gp140 Protein/Adjuvant HIV Vaccines in Two Immunization Regimes.Front Immunol. 2017 Feb 22;8:149. doi: 10.3389/fimmu.2017.00149. eCollection 2017. Front Immunol. 2017. PMID: 28275375 Free PMC article.
References
-
- Janeway CA, Jr., Travers P, Walport M. Immunobiology, the immune system in health and disease. New York, NY: Garland Publishing; 2005. et al.
-
- Hammarlund E, Lewis MW, Hansen SG. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. et al. - PubMed
-
- Berman PW, Gregory TJ, Riddle L. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990;345:622–625. et al. - PubMed
-
- Hu SL, Abrams K, Barber GN. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science. 1992;255:456–459. et al. - PubMed
-
- Belshe RB, Graham BS, Keefer MC. Neutralizing antibodies to HIV-1 in seronegative volunteers immunized with recombinant gp120 from the MN strain of HIV-1. JAMA. 1994;272:475–480. et al. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
