Host-pathogen interaction in invasive Salmonellosis
- PMID: 23055923
- PMCID: PMC3464234
- DOI: 10.1371/journal.ppat.1002933
Host-pathogen interaction in invasive Salmonellosis
Abstract
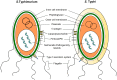
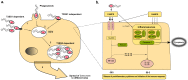
Salmonella enterica infections result in diverse clinical manifestations. Typhoid fever, caused by S. enterica serovar Typhi (S. Typhi) and S. Paratyphi A, is a bacteremic illness but whose clinical features differ from other Gram-negative bacteremias. Non-typhoidal Salmonella (NTS) serovars cause self-limiting diarrhea with occasional secondary bacteremia. Primary NTS bacteremia can occur in the immunocompromised host and infants in sub-Saharan Africa. Recent studies on host-pathogen interactions in Salmonellosis using genome sequencing, murine models, and patient studies have provided new insights. The full genome sequences of numerous S. enterica serovars have been determined. The S. Typhi genome, compared to that of S. Typhimurium, harbors many inactivated or disrupted genes. This can partly explain the different immune responses both serovars induce upon entering their host. Similar genome degradation is also observed in the ST313 S. Typhimurium strain implicated in invasive infection in sub-Saharan Africa. Virulence factors, most notably, type III secretion systems, Vi antigen, lipopolysaccharide and other surface polysaccharides, flagella, and various factors essential for the intracellular life cycle of S. enterica have been characterized. Genes for these factors are commonly carried on Salmonella Pathogenicity Islands (SPIs). Plasmids also carry putative virulence-associated genes as well as those responsible for antimicrobial resistance. The interaction of Salmonella pathogen-associated molecular patterns (PAMPs) with Toll-like receptors (TLRs) and NOD-like receptors (NLRs) leads to inflammasome formation, activation, and recruitment of neutrophils and macrophages and the production of pro-inflammatory cytokines, most notably interleukin (IL)-6, IL-1β, tumor necrosis factor (TNF)-α, and interferon-gamma (IFN)-γ. The gut microbiome may be an important modulator of this immune response. S. Typhimurium usually causes a local intestinal immune response, whereas S. Typhi, by preventing neutrophil attraction resulting from activation of TLRs, evades the local response and causes systemic infection. Potential new therapeutic strategies may lead from an increased understanding of infection pathogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Connor BA, Schwartz E (2005) Typhoid and paratyphoid fever in travellers. Lancet Infect Dis 5: 623–628. - PubMed
-
- Tsolis RM, Young GM, Solnick JV, Baumler AJ (2008) From bench to bedside: stealth of enteroinvasive pathogens. Nat Rev Microbiol 6: 883–892. - PubMed
-
- Beeching NJ, Parry CM (2011) Outpatient treatment of patients with enteric fever. Lancet Infect Dis 11: 419–421. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

