Promoted neuronal differentiation after activation of alpha4/beta2 nicotinic acetylcholine receptors in undifferentiated neural progenitors
- PMID: 23056257
- PMCID: PMC3464277
- DOI: 10.1371/journal.pone.0046177
Promoted neuronal differentiation after activation of alpha4/beta2 nicotinic acetylcholine receptors in undifferentiated neural progenitors
Abstract
Background: Neural progenitor is a generic term used for undifferentiated cell populations of neural stem, neuronal progenitor and glial progenitor cells with abilities for proliferation and differentiation. We have shown functional expression of ionotropic N-methyl-D-aspartate (NMDA) and gamma-aminobutyrate type-A receptors endowed to positively and negatively regulate subsequent neuronal differentiation in undifferentiated neural progenitors, respectively. In this study, we attempted to evaluate the possible functional expression of nicotinic acetylcholine receptor (nAChR) by undifferentiated neural progenitors prepared from neocortex of embryonic rodent brains.
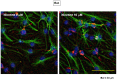
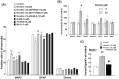
Methodology/principal findings: Reverse transcription polymerase chain reaction analysis revealed mRNA expression of particular nAChR subunits in undifferentiated rat and mouse progenitors prepared before and after the culture with epidermal growth factor under floating conditions. Sustained exposure to nicotine significantly inhibited the formation of neurospheres composed of clustered proliferating cells and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide reduction activity at a concentration range of 1 µM to 1 mM without affecting cell survival. In these rodent progenitors previously exposed to nicotine, marked promotion was invariably seen for subsequent differentiation into cells immunoreactive for a neuronal marker protein following the culture of dispersed cells under adherent conditions. Both effects of nicotine were significantly prevented by the heteromeric α4β2 nAChR subtype antagonists dihydro-β-erythroidine and 4-(5-ethoxy-3-pyridinyl)-N-methyl-(3E)-3-buten-1-amine, but not by the homomeric α7 nAChR subtype antagonist methyllycaconitine, in murine progenitors. Sustained exposure to nicotine preferentially increased the expression of Math1 among different basic helix-loop-helix proneural genes examined. In undifferentiated progenitors from embryonic mice defective of NMDA receptor subunit-1, nicotine was still effective in significantly inhibiting the proliferation.
Conclusions/significance: Functional α4β2 nAChR subtype would be constitutively expressed to play a role in the mechanism underlying the determination of proliferation and subsequent differentiation fate into a neuronal lineage in association with preferential promotion of Math1 expression in undifferentiated neural progenitors of developing rodent neocortex independently of NMDA receptor activation.
Conflict of interest statement
Figures


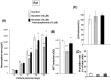






References
-
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97: 703–716. - PubMed
-
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, et al. (1999) Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96: 25–34. - PubMed
-
- Altman J, Das GD (1967) Postnatal neurogenesis in the guinea-pig. Nature 214: 1098–1101. - PubMed
-
- Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J (1998) Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol 36: 249–266. - PubMed
-
- Garcia-Verdugo JM, Doetsch F, Wichterle H, Lim DA, Alvarez-Buylla A (1998) Architecture and cell types of the adult subventricular zone: in search of the stem cells. J Neurobiol 36: 234–248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

