The alternative Epac/cAMP pathway and the MAPK pathway mediate hCG induction of leptin in placental cells
- PMID: 23056265
- PMCID: PMC3462743
- DOI: 10.1371/journal.pone.0046216
The alternative Epac/cAMP pathway and the MAPK pathway mediate hCG induction of leptin in placental cells
Abstract
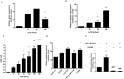
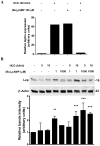
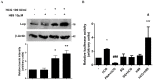
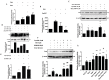
Pleiotropic effects of leptin have been identified in reproduction and pregnancy, particularly in the placenta, where it works as an autocrine hormone. In this work, we demonstrated that human chorionic gonadotropin (hCG) added to JEG-3 cell line or to placental explants induces endogenous leptin expression. We also found that hCG increased cAMP intracellular levels in BeWo cells in a dose-dependent manner, stimulated cAMP response element (CRE) activity and the cotransfection with an expression plasmid of a dominant negative mutant of CREB caused a significant inhibition of hCG stimulation of leptin promoter activity. These results demonstrate that hCG indeed activates cAMP/PKA pathway, and that this pathway is involved in leptin expression. Nevertheless, we found leptin induction by hCG is dependent on cAMP levels. Treatment with (Bu)(2)cAMP in combination with low and non stimulatory hCG concentrations led to an increase in leptin expression, whereas stimulatory concentrations showed the opposite effect. We found that specific PKA inhibition by H89 caused a significant increase of hCG leptin induction, suggesting that probably high cAMP levels might inhibit hCG effect. It was found that hCG enhancement of leptin mRNA expression involved the MAPK pathway. In this work, we demonstrated that hCG leptin induction through the MAPK signaling pathway is inhibited by PKA. We observed that ERK1/2 phosphorylation increased when hCG treatment was combined with H89. In view of these results, the involvement of the alternative cAMP/Epac signaling pathway was studied. We observed that a cAMP analogue that specifically activates Epac (CPT-OMe) stimulated leptin expression by hCG. In addition, the overexpression of Epac and Rap1 proteins increased leptin promoter activity and enhanced hCG. In conclusion, we provide evidence suggesting that hCG induction of leptin gene expression in placenta is mediated not only by activation of the MAPK signaling pathway but also by the alternative cAMP/Epac signaling pathway.
Conflict of interest statement
Figures

Similar articles
-
Regulation of placental leptin expression by cyclic adenosine 5'-monophosphate involves cross talk between protein kinase A and mitogen-activated protein kinase signaling pathways.Endocrinology. 2010 Aug;151(8):3738-51. doi: 10.1210/en.2010-0064. Epub 2010 May 19. Endocrinology. 2010. PMID: 20484458
-
Cross talk between cAMP and p38 MAPK pathways in the induction of leptin by hCG in human placental syncytiotrophoblasts.Reproduction. 2011 Aug;142(2):369-75. doi: 10.1530/REP-11-0053. Epub 2011 May 11. Reproduction. 2011. PMID: 21562093
-
hCG activates Epac-Erk1/2 signaling regulating Progesterone Receptor expression and function in human endometrial stromal cells.Mol Hum Reprod. 2017 Jun 1;23(6):393-405. doi: 10.1093/molehr/gax015. Mol Hum Reprod. 2017. PMID: 28333280
-
cAMP Signaling in Cancer: A PKA-CREB and EPAC-Centric Approach.Cells. 2022 Jun 24;11(13):2020. doi: 10.3390/cells11132020. Cells. 2022. PMID: 35805104 Free PMC article. Review.
-
Cyclic AMP sensor EPAC proteins and energy homeostasis.Trends Endocrinol Metab. 2014 Feb;25(2):60-71. doi: 10.1016/j.tem.2013.10.004. Epub 2013 Nov 12. Trends Endocrinol Metab. 2014. PMID: 24231725 Free PMC article. Review.
Cited by
-
The road to ERK activation: Do neurons take alternate routes?Cell Signal. 2020 Apr;68:109541. doi: 10.1016/j.cellsig.2020.109541. Epub 2020 Jan 13. Cell Signal. 2020. PMID: 31945453 Free PMC article. Review.
-
Role of Exchange Protein Directly Activated by Cyclic AMP Isoform 1 in Energy Homeostasis: Regulation of Leptin Expression and Secretion in White Adipose Tissue.Mol Cell Biol. 2016 Sep 12;36(19):2440-50. doi: 10.1128/MCB.01034-15. Print 2016 Oct 1. Mol Cell Biol. 2016. PMID: 27381457 Free PMC article.
-
Maternal Body Weight and Gestational Diabetes Differentially Influence Placental and Pregnancy Outcomes.J Clin Endocrinol Metab. 2016 Jan;101(1):59-68. doi: 10.1210/jc.2015-2590. Epub 2015 Oct 29. J Clin Endocrinol Metab. 2016. PMID: 26513002 Free PMC article.
-
Requirement for protein kinase A in the phosphorylation of the TGFβ receptor-interacting protein km23-1 as a component of TGFβ downstream effects.Exp Cell Res. 2013 Apr 1;319(6):897-907. doi: 10.1016/j.yexcr.2012.12.029. Epub 2013 Jan 16. Exp Cell Res. 2013. PMID: 23333499 Free PMC article.
-
The cGMP system in normal and degenerating mouse neuroretina: New proteins with cGMP interaction potential identified by a proteomics approach.J Neurochem. 2021 Jun;157(6):2173-2186. doi: 10.1111/jnc.15251. Epub 2020 Dec 5. J Neurochem. 2021. PMID: 33230839 Free PMC article.
References
-
- Fitzgerald JS, Busch S, Wengenmayer T, Foerster K, de la Motte T, et al. (2005) Signal transduction in trophoblast invasion. Chem Immunol Allergy 88: 181–199. - PubMed
-
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, et al. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432. - PubMed
-
- Houseknecht KL, Portocarrero CP (1998) Leptin and its receptors: regulators of whole-body energy homeostasis. Domest Anim Endocrinol 15: 457–475. - PubMed
-
- Reitman ML, Bi S, Marcus-Samuels B, Gavrilova O (2001) Leptin and its role in pregnancy and fetal development–an overview. Biochem Soc Trans 29: 68–72. - PubMed
-
- Henson MC, Castracane VD (2005) Leptin in Pregnancy: An Update. Biol Reprod. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

