Inactivation of the Celf1 gene that encodes an RNA-binding protein delays the first wave of spermatogenesis in mice
- PMID: 23056285
- PMCID: PMC3462782
- DOI: 10.1371/journal.pone.0046337
Inactivation of the Celf1 gene that encodes an RNA-binding protein delays the first wave of spermatogenesis in mice
Abstract
Background: The first wave of spermatogenesis in mammals is characterized by a sequential and synchronous appearance of germ cells in the prepubertal testis. Post-transcriptional controls of gene expression play important roles in this process but the molecular actors that underlie them are poorly known.
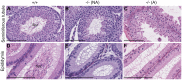
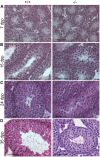
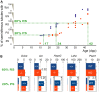
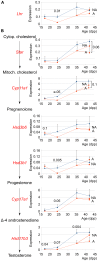
Methodology/principal findings: We evaluated the requirement for the RNA-binding protein CELF1 during the first wave of spermatogenesis in mice. Mice inactivated for Celf1 gene were not viable on pure genetic backgrounds. On a mixed background, we observed by histology and gene profiling by RT-qPCR that the testes of inactivated prepubertal mice were characterized by several features. (i) Spermiogenesis (differentiation of post-meiotic cells) was blocked in a subset of prepubertal inactivated mice. (ii) The appearance of the different stages of germ cell development was delayed by several days. (iii) The expression of markers of Leydig cells functions was similarly delayed.
Conclusions/significance: Celf1 disruption is responsible for a blockage of spermiogenesis both in adults and in prepubertal males. Hence, the spermiogenesis defects found in Celf1-inactivated adults appear from the first wave of spermiogenesis. The disruption of Celf1 gene is also responsible for a fully penetrant delayed first wave of spermatogenesis, and a delay of steroidogenesis may be the cause for the delay of germ cells differentiation.
Conflict of interest statement
Figures
Similar articles
-
Hypogonadism Associated with Cyp19a1 (Aromatase) Posttranscriptional Upregulation in Celf1 Knockout Mice.Mol Cell Biol. 2015 Sep;35(18):3244-53. doi: 10.1128/MCB.00074-15. Epub 2015 Jul 13. Mol Cell Biol. 2015. PMID: 26169831 Free PMC article.
-
Inactivation of CUG-BP1/CELF1 causes growth, viability, and spermatogenesis defects in mice.Mol Cell Biol. 2007 Feb;27(3):1146-57. doi: 10.1128/MCB.01009-06. Epub 2006 Nov 27. Mol Cell Biol. 2007. PMID: 17130239 Free PMC article.
-
E2F1 controls germ cell apoptosis during the first wave of spermatogenesis.Andrology. 2015 Sep;3(5):1000-14. doi: 10.1111/andr.12090. Andrology. 2015. PMID: 26311345 Free PMC article.
-
Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice.Endocr Rev. 2009 Apr;30(2):119-32. doi: 10.1210/er.2008-0025. Epub 2009 Jan 27. Endocr Rev. 2009. PMID: 19176467 Free PMC article. Review.
-
Role of RNA-binding proteins in mammalian spermatogenesis.Int J Androl. 2010 Feb;33(1):2-12. doi: 10.1111/j.1365-2605.2009.00959.x. Epub 2009 Mar 5. Int J Androl. 2010. PMID: 19281489 Review.
Cited by
-
CELFish ways to modulate mRNA decay.Biochim Biophys Acta. 2013 Jun-Jul;1829(6-7):695-707. doi: 10.1016/j.bbagrm.2013.01.001. Epub 2013 Jan 15. Biochim Biophys Acta. 2013. PMID: 23328451 Free PMC article. Review.
-
In-utero exposure to real-life environmental chemicals disrupts gene expression within the hypothalamo-pituitary-gonadal axis of prepubertal and adult rams.Environ Res. 2025 Jan 1;264(Pt 1):120303. doi: 10.1016/j.envres.2024.120303. Epub 2024 Nov 5. Environ Res. 2025. PMID: 39510237 Free PMC article.
-
Alternative RNA Splicing in the Pathogenesis of Liver Disease.Front Endocrinol (Lausanne). 2017 Jun 21;8:133. doi: 10.3389/fendo.2017.00133. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28680417 Free PMC article. Review.
-
Hypogonadism Associated with Cyp19a1 (Aromatase) Posttranscriptional Upregulation in Celf1 Knockout Mice.Mol Cell Biol. 2015 Sep;35(18):3244-53. doi: 10.1128/MCB.00074-15. Epub 2015 Jul 13. Mol Cell Biol. 2015. PMID: 26169831 Free PMC article.
-
CUG-BP, Elav-like family member 1 (CELF1) is required for normal myofibrillogenesis, morphogenesis, and contractile function in the embryonic heart.Dev Dyn. 2016 Aug;245(8):854-73. doi: 10.1002/dvdy.24413. Epub 2016 May 31. Dev Dyn. 2016. PMID: 27144987 Free PMC article.
References
-
- Russell L, Ettlin R, SinhaHikim A, Clegg E (1990) Histological and histopathological evaluation of the testis. St Louis: Cache River Press.
-
- Itman C, Mendis S, Barakat B, Loveland KL (2006) All in the family: TGF-beta family action in testis development. Reproduction 132: 233–246. - PubMed
-
- Shima JE, McLean DJ, McCarrey JR, Griswold MD (2004) The murine testicular transcriptome: characterizing gene expression in the testis during the progression of spermatogenesis. Biol Reprod 71: 319–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

