MultiDsk: a ubiquitin-specific affinity resin
- PMID: 23056298
- PMCID: PMC3463603
- DOI: 10.1371/journal.pone.0046398
MultiDsk: a ubiquitin-specific affinity resin
Abstract
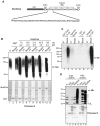
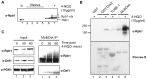
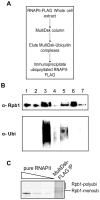
Ubiquitylation is a highly diverse and complex post-translational modification for the regulation of protein function and stability. Studies of ubiquitylation have, however, been hampered by its rapid reversal in cell extracts, for example through the action of de-ubiquitylating enzymes (DUBs). Here we describe a novel ubiquitin-binding protein reagent, MultiDsk, composed of an array of five UBA domains from the yeast ubiquitin-binding protein Dsk2, fused to GST. MultiDsk binds ubiquitylated substrates with unprecedented avidity, and can be used as both an affinity resin to study protein ubiquitylation, and to effectively protect ubiquitylated proteins from the action of DUBs and the proteasome in crude cell extracts. We use the resin to show that the Def1 protein becomes ubiquitylated in response to DNA damage, and to isolate ubiquitylated forms of RNA polymerase II.
Conflict of interest statement
Figures

References
-
- Hershko A, Eytan E, Ciechanover A, Haas AL (1982) Immunochemical analysis of the turnover of ubiquitin-protein conjugates in intact cells. Relationship to the breakdown of abnormal proteins. J Biol Chem 257: 13964–13970. - PubMed
-
- Ulrich HD, Walden H (2010) Ubiquitin signalling in DNA replication and repair. Nat Rev Mol Cell Biol 11: 479–489. - PubMed
-
- Staub O, Rotin D (2006) Role of ubiquitylation in cellular membrane transport. Physiol Rev 86: 669–707. - PubMed
-
- Nagy V, Dikic I (2010) Ubiquitin ligase complexes: from substrate selectivity to conjugational specificity. Biological Chemistry 391: 163–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

