Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells
- PMID: 23056368
- PMCID: PMC3464294
- DOI: 10.1371/journal.pone.0046609
Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells
Abstract
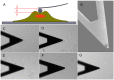
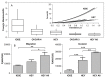
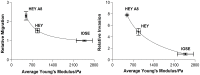
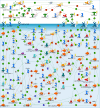
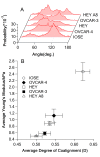
The metastatic potential of cells is an important parameter in the design of optimal strategies for the personalized treatment of cancer. Using atomic force microscopy (AFM), we show, consistent with previous studies conducted in other types of epithelial cancer, that ovarian cancer cells are generally softer and display lower intrinsic variability in cell stiffness than non-malignant ovarian epithelial cells. A detailed examination of highly invasive ovarian cancer cells (HEY A8) relative to their less invasive parental cells (HEY), demonstrates that deformability is also an accurate biomarker of metastatic potential. Comparative gene expression analyses indicate that the reduced stiffness of highly metastatic HEY A8 cells is associated with actin cytoskeleton remodeling and microscopic examination of actin fiber structure in these cell lines is consistent with this prediction. Our results indicate that cell stiffness may be a useful biomarker to evaluate the relative metastatic potential of ovarian and perhaps other types of cancer cells.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

