Bimodal activation of different neuron classes with the spectrally red-shifted channelrhodopsin chimera C1V1 in Caenorhabditis elegans
- PMID: 23056472
- PMCID: PMC3463556
- DOI: 10.1371/journal.pone.0046827
Bimodal activation of different neuron classes with the spectrally red-shifted channelrhodopsin chimera C1V1 in Caenorhabditis elegans
Abstract
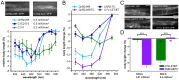
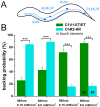
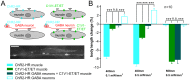
The C. elegans nervous system is particularly well suited for optogenetic analyses of circuit function: Essentially all connections have been mapped, and light can be directed at the neuron of interest in the freely moving, transparent animals, while behavior is observed. Thus, different nodes of a neuronal network can be probed for their role in controlling a particular behavior, using different optogenetic tools for photo-activation or -inhibition, which respond to different colors of light. As neurons may act in concert or in opposing ways to affect a behavior, one would further like to excite these neurons concomitantly, yet independent of each other. In addition to the blue-light activated Channelrhodopsin-2 (ChR2), spectrally red-shifted ChR variants have been explored recently. Here, we establish the green-light activated ChR chimera C1V1 (from Chlamydomonas and Volvox ChR1's) for use in C. elegans. We surveyed a number of red-shifted ChRs, and found that C1V1-ET/ET (E122T; E162T) works most reliable in C. elegans, with 540-580 nm excitation, which leaves ChR2 silent. However, as C1V1-ET/ET is very light sensitive, it still becomes activated when ChR2 is stimulated, even at 400 nm. Thus, we generated a highly efficient blue ChR2, the H134R; T159C double mutant (ChR2-HR/TC). Both proteins can be used in the same animal, in different neurons, to independently control each cell type with light, enabling a further level of complexity in circuit analyses.
Conflict of interest statement
Figures

References
-
- Nagel G, Szellas T, Kateriya S, Adeishvili N, Hegemann P, et al. (2005) Channelrhodopsins: directly light-gated cation channels. Biochem Soc Trans 33: 863–866. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

