Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: molecular phenotype of chemoresistant ovarian tumors
- PMID: 23056490
- PMCID: PMC3466197
- DOI: 10.1371/journal.pone.0046858
Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: molecular phenotype of chemoresistant ovarian tumors
Abstract
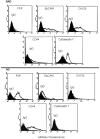
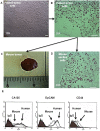
Tumor cells in ascites are a major source of disease recurrence in ovarian cancer patients. In an attempt to identify and profile the population of ascites cells obtained from ovarian cancer patients, a novel method was developed to separate adherent (AD) and non-adherent (NAD) cells in culture. Twenty-five patients were recruited to this study; 11 chemonaive (CN) and 14 chemoresistant (CR). AD cells from both CN and CR patients exhibited mesenchymal morphology with an antigen profile of mesenchymal stem cells and fibroblasts. Conversely, NAD cells had an epithelial morphology with enhanced expression of cancer antigen 125 (CA125), epithelial cell adhesion molecule (EpCAM) and cytokeratin 7. NAD cells developed infiltrating tumors and ascites within 12-14 weeks after intraperitoneal (i.p.) injections into nude mice, whereas AD cells remained non-tumorigenic for up to 20 weeks. Subsequent comparison of selective epithelial, mesenchymal and cancer stem cell (CSC) markers between AD and NAD populations of CN and CR patients demonstrated an enhanced trend in mRNA expression of E-cadherin, EpCAM, STAT3 and Oct4 in the NAD population of CR patients. A similar trend of enhanced mRNA expression of CD44, MMP9 and Oct4 was observed in the AD population of CR patients. Hence, using a novel purification method we demonstrate for the first time a distinct separation of ascites cells into epithelial tumorigenic and mesenchymal non-tumorigenic populations. We also demonstrate that cells from the ascites of CR patients are predominantly epithelial and show a trend towards increased mRNA expression of genes associated with CSCs, compared to cells isolated from the ascites of CN patients. As the tumor cells in the ascites of ovarian cancer patients play a dominant role in disease recurrence, a thorough understanding of the biology of the ascites microenvironment from CR and CN patients is essential for effective therapeutic interventions.
Conflict of interest statement
Figures











References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

