Phase I randomized safety study of twice daily dosing of acidform vaginal gel: candidate antimicrobial contraceptive
- PMID: 23056520
- PMCID: PMC3466198
- DOI: 10.1371/journal.pone.0046901
Phase I randomized safety study of twice daily dosing of acidform vaginal gel: candidate antimicrobial contraceptive
Abstract
Background: Acidform gel, an acid-buffering product that inactivates spermatozoa, may be an effective topical non-hormonal contraceptive. This study was designed to evaluate the safety of vaginal dosing and effects of Acidform on mucosal immune mediators, antimicrobial properties of genital secretions, and vaginal microbiota.
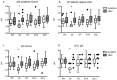
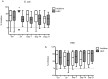
Methods: Thirty-six sexually abstinent U.S. women were randomized to apply Acidform or hydroxyethylcellulose (HEC) placebo gel twice daily for 14 consecutive days. Safety was assessed by symptoms and pelvic examination. The impact of gel on mucosal immunity was assessed by quantifying cytokines, chemokines, antimicrobial proteins and antimicrobial activity of genital secretions collected by cervicovaginal lavage (CVL) at screening, 2 hours after gel application, and on days 7, 14 and 21. Vaginal microbiota was characterized at enrollment and day 14 using species-specific quantitative PCR assays.
Results: The median vaginal and cervical pH was significantly lower 2 hours after application of Acidform and was associated with an increase in the bactericidal activity of CVL against E. coli. However, 65% of women who received Acidform had at least one local adverse event compared with 11% who received placebo (p = 0.002). While there was no increase in inflammatory cytokines or chemokines, CVL concentrations of lactoferrin and interleukin-1 receptor antagonist (IL-1ra), an anti-inflammatory protein, were significantly lower following Acidform compared to HEC placebo gel application. There were no significant changes in Lactobacillus crispatus or Lactobacillus jensenii in either group but there was a decrease in Gardnerella vaginalis in the Acidform group (p = 0.08).
Conclusions: Acidform gel may augment mucosal defense as evidenced by an increase in bactericidal activity of genital secretions against E. coli and a decrease in Gardnerella vaginalis colonization. However, Acidform was associated with more irritation than placebo and lower levels of antimicrobial (lactoferrin) and anti-inflammatory (IL-1ra) proteins. These findings indicate the need for additional safety studies of this candidate non-hormonal contraceptive.
Trial registration: ClinicalTrials.gov NCT00850837.
Conflict of interest statement
Figures
References
-
- Lavreys L, Baeten JM, Martin HL Jr, Overbaugh J, Mandaliya K, et al. (2004) Hormonal contraception and risk of HIV-1 acquisition: results of a 10-year prospective study. AIDS 18: 695–697. - PubMed
-
- Martin HL Jr, Nyange PM, Richardson BA, Lavreys L, Mandaliya K, et al. (1998) Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. The Journal of infectious diseases 178: 1053–1059. - PubMed
-
- Roddy RE, Zekeng L, Ryan KA, Tamoufe U, Tweedy KG (2002) Effect of nonoxynol-9 gel on urogenital gonorrhea and chlamydial infection: a randomized controlled trial. JAMA : the journal of the American Medical Association 287: 1117–1122. - PubMed
-
- Roddy RE, Zekeng L, Ryan KA, Tamoufe U, Weir SS, et al. (1998) A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. The New England journal of medicine 339: 504–510. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

