Aphid gel saliva: sheath structure, protein composition and secretory dependence on stylet-tip milieu
- PMID: 23056521
- PMCID: PMC3462764
- DOI: 10.1371/journal.pone.0046903
Aphid gel saliva: sheath structure, protein composition and secretory dependence on stylet-tip milieu
Abstract
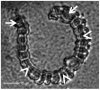
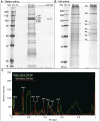
In order to separate and analyze saliva types secreted during stylet propagation and feeding, aphids were fed on artificial diets. Gel saliva was deposited as chains of droplets onto Parafilm membranes covering the diets into which watery saliva was secreted. Saliva compounds collected from the diet fluid were separated by SDS-PAGE, while non-soluble gel saliva deposits were processed in a novel manner prior to protein separation by SDS-PAGE. Soluble (watery saliva) and non-soluble (gel saliva) protein fractions were significantly different. To test the effect of the stylet milieu on saliva secretion, aphids were fed on various diets. Hardening of gel saliva is strongly oxygen-dependent, probably owing to formation of sulfide bridges by oxidation of sulphydryl groups. Surface texture of gel saliva deposits is less pronounced under low-oxygen conditions and disappears in dithiothreitol containing diet. Using diets mimicking sieve-element sap and cell-wall fluid respectively showed that the soluble protein fraction was almost exclusively secreted in sieve elements while non-soluble fraction was preferentially secreted at cell wall conditions. This indicates that aphids are able to adapt salivary secretion in dependence of the stylet milieu.
Conflict of interest statement
Figures


References
-
- Hewer A, Will T, van Bel AJE (2010) Plant cues for aphid navigation in vascular tissues. J Exp Biol 213: 4030–4042. - PubMed
-
- Hewer A, Becker A, van Bel AJE (2011) An aphid's Odyssey. The cortical quest for the vascular bundle. J Exp Biol 214: 3868–3879. - PubMed
-
- Martin B, Collar JL, Tjallingii WF, Fereres A (1997) Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J Gen Virol 78: 2701–2705. - PubMed
-
- Powell G (2005) Intracellular salivation is the aphid activity associated with inoculation of non-persistently transmitted viruses. J Gen Virol 86: 469–472. - PubMed
-
- Ponsen MB (1972) The site of potato leafroll virus multiplication in its vector, Myzus persicae . Mededelingen van de Landbouwhogeschool Wageningen 16: 1–147.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

