Cultivation and characterization of cornea limbal epithelial stem cells on lens capsule in animal material-free medium
- PMID: 23056608
- PMCID: PMC3467238
- DOI: 10.1371/journal.pone.0047187
Cultivation and characterization of cornea limbal epithelial stem cells on lens capsule in animal material-free medium
Abstract
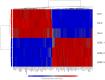
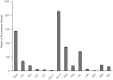
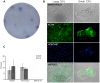
A simple, reproducible, animal-material free method for cultivating and characterizing cornea limbal epithelial stem cells (LESCs) on human lens capsule (LC) was developed for future clinical transplantation. The limbal tissue explants (2 × 2 × 0.25 mm) were harvested from 77 cadavers and expanded ex vivo on either cell culture plates or LC in medium containing human serum as the only growth supplement. Cell outgrowth at the edge of the explants was observed within 24 hours of cultivation and achieved viable outgrowth (>97% viability as measured by MTT assay and flow cytometry) within two weeks. The outgrowing cells were examined by genome-wide microarray including markers of stemness (p63α, ABCG2, CK19, Vimentin and Integrin α9), proliferation (Ki-67), limbal epithelial cells (CK 8/18 and 14) and differentiated cornea epithelial cells (CK 3 and 12). Immunostaining revealed the non-hematopoietic, -endothelial and -mesenchymal stem cell phenotype of the LESCs and the localization of specific markers in situ. Cell adhesion molecules, integrins and lectin-based surface carbohydrate profiling showed a specific pattern on these cells, while colony-formation assay confirmed their clonal potency. The LESCs expressed a specific surface marker fingerprint (CD117/c-kit, CXCR4, CD144/VE-Cadherin, CD146/MCAM, CD166/ALCAM, and surface carbohydrates: WGA, ConA, RCA, PNA and AIL) which can be used for better localization of the limbal stem cell niche. In summary, we report a novel method combining the use of a medium with human serum as the only growth supplement with LC for cultivating, characterizing and expanding cornea LESCs from cadavers or alternatively from autologous donors for possible treatment of LESC deficiency.
Conflict of interest statement
Figures


References
-
- Davanger M, Evensen A (1971) Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 229: 560–561. - PubMed
-
- Tseng SC (1989) Concept and application of limbal stem cells. Eye (Lond) 3 (Pt 2): 141–157. - PubMed
-
- Schlötzer-Schrehardt U, Kruse FE (2005) Identification and characterization of limbal stem cells. Exp Eye Res 81: 247–264. - PubMed
-
- Notara M, Alatza A, Gilfillan J, Harris AR, Levis HJ, et al. (2010) In sickness and in health: Corneal epithelial stem cell biology, pathology and therapy. Exp Eye Res 90: 188–195. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

