H11/HspB8 and Its Herpes Simplex Virus Type 2 Homologue ICP10PK Share Functions That Regulate Cell Life/Death Decisions and Human Disease
- PMID: 23056924
- PMCID: PMC3463903
- DOI: 10.1155/2012/395329
H11/HspB8 and Its Herpes Simplex Virus Type 2 Homologue ICP10PK Share Functions That Regulate Cell Life/Death Decisions and Human Disease
Abstract
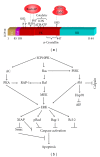
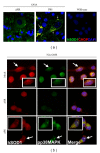
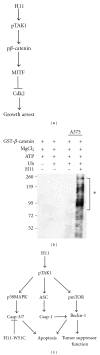
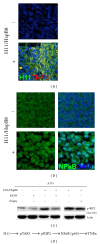
Small heat shock proteins (sHsp) also known as HspB are a large family of widely expressed proteins that contain a 90 residues domain known as α-crystallin. Here, we focus on the family member H11/HspB8 and its herpes simplex virus type 2 (HSV-2) homologue ICP10PK, and discuss the possible impact of this relationship on human disease. H11/HspB8 and ICP10PK are atypical protein kinases. They share multi-functional activity that encompasses signaling, unfolded protein response (UPR) and the regulation of life cycle potential. In melanocytes H11/HspB8 causes growth arrest. It is silenced in a high proportion of melanoma prostate cancer, Ewing's sarcoma and hematologic malignancies through aberrant DNA methylation. Its restored expression induces cell death and inhibits tumor growth in xenograft models, identifying H11/HspB8 as a tumor suppressor. This function involves the activation of multiple and distinct death pathways, all of which initiate with H11/HspB8-mediated phosphorylation of transforming growth factor β-activated kinase 1 (TAK1). Both ICP10PK and H11/HspB8 were implicated in inflammatory processes that involve dendritic cells activation through Toll-like receptor-dependent pathways and may contribute to the onset of autoimmunity. The potential evolutionary relationship of H11/HspB8 to ICP10PK, its impact on human disorders and the development of therapeutic strategies are discussed.
Figures
References
-
- Hartl FU. Chaperone-assisted protein folding: the path to discovery from a personal perspective. Nature Medicine. 2011;17:1206–1210. - PubMed
-
- Mymrikov EV, Seit-Nebi AS, Gusev NB. Large potentials of small heat shock proteins. Physiological Reviews. 2011;91:1123–1159. - PubMed
-
- Acunzo J, Katsogiannou M, Rocchi P. Small heat shock proteins HSP27 (HspB1), αB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. International Journal of Biochemistry & Cell Biology. 2012;44(10):1622–1631. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

