A randomized trial to investigate the effects of pre-natal and infant nutritional supplementation on infant immune development in rural Gambia: the ENID trial: Early Nutrition and Immune Development
- PMID: 23057665
- PMCID: PMC3534399
- DOI: 10.1186/1471-2393-12-107
A randomized trial to investigate the effects of pre-natal and infant nutritional supplementation on infant immune development in rural Gambia: the ENID trial: Early Nutrition and Immune Development
Abstract
Background: Recent observational research indicates that immune development may be programmed by nutritional exposures early in life. Such findings require replication from trials specifically designed to assess the impact of nutritional intervention during pregnancy on infant immune development. The current trial seeks to establish: (a) which combination of protein-energy (PE) and multiple-micronutrient (MMN) supplements would be most effective; and (b) the most critical periods for intervention in pregnancy and infancy, for optimal immune development in infancy.
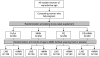
Methods/design: The ENID Trial is a 2 x 2 x 2 factorial randomized, partially blind trial to assess whether nutritional supplementation to pregnant women (from < 20 weeks gestation to term) and their infants (from 6 to 12 months of age) can enhance infant immune development. Eligible pregnant women from the West Kiang region of The Gambia (pregnancy dated by ultrasound examination) are randomized on entry to 4 intervention groups (Iron-folate (FeFol = standard care), multiple micronutrients (MMN), protein-energy (PE), PE + MMN). Women are visited at home weekly for supplement administration and morbidity assessment and seen at MRC Keneba at 20 and 30 weeks gestation for a detailed antenatal examination, including ultrasound. At delivery, cord blood and placental samples are collected, with detailed infant anthropometry collected within 72 hours. Infants are visited weekly thereafter for a morbidity questionnaire. From 6 to 12 months of age, infants are further randomized to a lipid-based nutritional supplement, with or without additional MMN. The primary outcome measures of this study are thymic development during infancy, and antibody response to vaccination. Measures of cellular markers of immunity will be made in a selected sub-cohort. Subsidiary studies to the main trial will additionally assess the impact of supplementation on infant growth and development to 24 months of age.
Discussion: The proposed trial is designed to test whether nutritional repletion can enhance early immune development and, if so, to help determine the most efficacious form of nutritional support. Where there is evidence of benefit from a specific intervention/combination of interventions, future research should focus on refining the supplements to achieve the optimal, most cost-effective balance of interventions for improved health outcomes.
References
-
- Barker DJP. Mothers, Babies and Diseases in Later Life. London: BMJ Publishing Group; 1994.
-
- Prentice AM, Whitehead RG, Roberts SB, Paul AA. Long-term energy balance in childbearing Gambian women. Am J Clin Nutr. 1981;34:2790–2799. - PubMed
-
- Rayco-Solon P, Fulford AJ, Prentice AM. Differential effects of seasonality on preterm birth and intrauterine growth restriction in rural Africans. Am J Clin Nutr. 2005;81(1):134–139. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical