A bacterial hemerythrin domain regulates the activity of a Vibrio cholerae diguanylate cyclase
- PMID: 23057727
- PMCID: PMC3549010
- DOI: 10.1021/bi3011797
A bacterial hemerythrin domain regulates the activity of a Vibrio cholerae diguanylate cyclase
Abstract
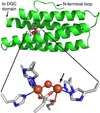
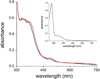
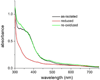
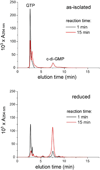
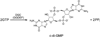
The first demonstrated example of a regulatory function for a bacterial hemerythrin (Bhr) domain is reported. Bhrs have a characteristic sequence motif providing ligand residues for a type of non-heme diiron site that is known to bind O(2) and undergo autoxidation. The amino acid sequence encoded by the VC1216 gene from Vibrio cholerae O1 biovar El Tor str. N16961 contains an N-terminal Bhr domain connected to a C-terminal domain characteristic of bacterial diguanylate cyclases (DGCs) that catalyze formation of cyclic di-(3',5')-guanosine monophosphate (c-di-GMP) from GTP. This protein, Vc Bhr-DGC, was found to contain two tightly bound non-heme iron atoms per protein monomer. The as-isolated protein showed the spectroscopic signatures of oxo/dicarboxylato-bridged non-heme diferric sites of previously characterized Bhr domains. The diiron site was capable of cycling between diferric and diferrous forms, the latter of which was stable only under anaerobic conditions, undergoing rapid autoxidation upon being exposed to air. Vc Bhr-DGC showed approximately 10 times higher DGC activity in the diferrous than in the diferric form. The level of intracellular c-di-GMP is known to regulate biofilm formation in V. cholerae. The higher DGC activity of the diferrous Vc Bhr-DGC is consistent with induction of biofilm formation in low-dioxygen environments. The non-heme diiron cofactor in the Bhr domain thus represents an alternative to heme or flavin for redox and/or diatomic gas sensing and regulation of DGC activity.
Figures



References
-
- Romling U, Gomelsky M, Galperin MY. C-di-GMP: the dawning of a novel bacterial signalling system. Mol. Microbiol. 2005;57:629–639. - PubMed
-
- Hengge R. Principles of c-di-GMP signalling in bacteria. Nature Rev. Microbiol. 2009;7:263–273. - PubMed
-
- Wolfe AJ, Visick KL, editors. The second messenger cyclic di-GMP. Washington, DC: ASM Press; 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

