Vincristine enhances amoeboid-like motility via GEF-H1/RhoA/ROCK/Myosin light chain signaling in MKN45 cells
- PMID: 23057787
- PMCID: PMC3522013
- DOI: 10.1186/1471-2407-12-469
Vincristine enhances amoeboid-like motility via GEF-H1/RhoA/ROCK/Myosin light chain signaling in MKN45 cells
Abstract
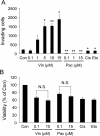
Background: Anti-cancer drugs are widely used in cancer treatment frequently combined with surgical therapy and/or radiation therapy. Although surgery and radiation have been suggested to facilitate invasion and metastasis of tumor cells in some cases, there is so far little information about the effect of anti-cancer drugs on cellular invasive ability and metastasis. In this study, using four different anti-cancer drugs (vincristine, paclitaxel, cisplatin and etoposide), we examined whether these drugs influence the invasive ability of tumor cells.
Methods: Human gastric adenocarcinoma MKN45 cells were used to evaluate the effect of anti-cancer drugs. After drug treatment, cellular invasive ability was assessed using the Matrigel invasion chamber. Cytoskeletal changes after treatment were examined microscopically with F-actin staining. In addition, we monitored cellular motility in 3D matrigel environment by time-lapse microscopic analysis. The drug-induced activation of RhoA and ROCK was evaluated by pull-down assay and Western blotting using an antibody against phosphorylated myosin light chain (MLC), respectively. Where necessary, a ROCK inhibitor Y27632 and siRNA for guanine nucleotide exchange factor-H1 (GEF-H1) were applied.
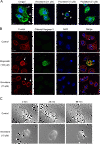
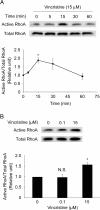
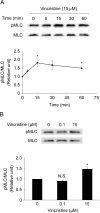
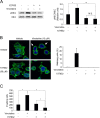
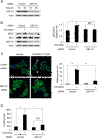
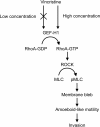
Results: Among all drugs tested, only vincristine stimulated the invasive ability of MKN45 cells. Microscopic analysis revealed that vincristine induced the formation of non-apoptotic membrane blebs and amoeboid-like motility. Vincristine significantly enhanced RhoA activity and MLC phosphorylation, suggesting the involvement of RhoA/ROCK pathway in the vincristine-induced cytoskeletal reorganization and cellular invasion. Furthermore, we found that Y27632 as well as the siRNA for GEF-H1, a RhoA-specific activator, attenuated MLC phosphorylation, the formation of membrane blebs and the invasive ability after vincristine treatment.
Conclusions: These results indicate that vincristine activates GEF-H1/RhoA/ROCK/MLC signaling, thereby promoting amoeboid-like motility and the invasive ability of MKN45 cells.
Figures
Similar articles
-
Vincristine promotes migration and invasion of colorectal cancer HCT116 cells through RhoA/ROCK/ Myosin light chain pathway.Cell Mol Biol (Noisy-le-grand). 2016 Oct 31;62(12):91-96. doi: 10.14715/cmb/2016.62.12.16. Cell Mol Biol (Noisy-le-grand). 2016. PMID: 27894406
-
GEF-H1 couples nocodazole-induced microtubule disassembly to cell contractility via RhoA.Mol Biol Cell. 2008 May;19(5):2147-53. doi: 10.1091/mbc.e07-12-1269. Epub 2008 Feb 20. Mol Biol Cell. 2008. PMID: 18287519 Free PMC article.
-
MiR-31 Regulates Rho-Associated Kinase-Myosin Light Chain (ROCK-MLC) Pathway and Inhibits Gastric Cancer Invasion: Roles of RhoA.Med Sci Monit. 2016 Dec 1;22:4679-4691. doi: 10.12659/msm.898399. Med Sci Monit. 2016. PMID: 27904131 Free PMC article.
-
Rho/ROCK signaling in motility and metastasis of gastric cancer.World J Gastroenterol. 2014 Oct 14;20(38):13756-66. doi: 10.3748/wjg.v20.i38.13756. World J Gastroenterol. 2014. PMID: 25320513 Free PMC article. Review.
-
The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.Cell Signal. 2014 Mar;26(3):483-91. doi: 10.1016/j.cellsig.2013.11.034. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308970 Review.
Cited by
-
Trading in your spindles for blebs: the amoeboid tumor cell phenotype in prostate cancer.Asian J Androl. 2014 Jul-Aug;16(4):530-5. doi: 10.4103/1008-682X.122877. Asian J Androl. 2014. PMID: 24589458 Free PMC article.
-
The emerging role of microtubules in invasion plasticity.Front Oncol. 2023 Feb 13;13:1118171. doi: 10.3389/fonc.2023.1118171. eCollection 2023. Front Oncol. 2023. PMID: 36860323 Free PMC article. Review.
-
Movers and shakers: cell cytoskeleton in cancer metastasis.Br J Pharmacol. 2014 Dec;171(24):5507-23. doi: 10.1111/bph.12704. Epub 2014 Jul 2. Br J Pharmacol. 2014. PMID: 24665826 Free PMC article. Review.
-
Activation of neural lineage networks and ARHGEF2 in enzalutamide-resistant and neuroendocrine prostate cancer and association with patient outcomes.Commun Med (Lond). 2022 Sep 21;2:118. doi: 10.1038/s43856-022-00182-9. eCollection 2022. Commun Med (Lond). 2022. PMID: 36159187 Free PMC article.
-
MMP inhibitor Ilomastat induced amoeboid-like motility via activation of the Rho signaling pathway in glioblastoma cells.Tumour Biol. 2016 Dec;37:16177–16186. doi: 10.1007/s13277-016-5464-5. Epub 2016 Oct 14. Tumour Biol. 2016. PMID: 27743382
References
-
- Ben-Eliyahu S. The promotion of tumor metastasis by surgery and stress: immunological basis and implications for psychoneuroimmunology. Brain Behav Immun. 2003;17(Suppl 1):S27–S36. - PubMed
-
- Goldfarb Y, Ben-Eliyahu S. Surgery as a risk factor for breast cancer recurrence and metastasis: mediating mechanisms and clinical prophylactic approaches. Breast Dis. 2006;26:99–114. - PubMed
-
- Kaliski A, Maggiorella L, Cengel KA, Mathe D, Rouffiac V, Opolon P, Lassau N, Bourhis J, Deutsch E. Angiogenesis and tumor growth inhibition by a matrix metalloproteinase inhibitor targeting radiation-induced invasion. Mol Cancer Ther. 2005;4(11):1717–1728. doi: 10.1158/1535-7163.MCT-05-0179. - DOI - PubMed
-
- Zhai GG, Malhotra R, Delaney M, Latham D, Nestler U, Zhang M, Mukherjee N, Song Q, Robe P, Chakravarti A. Radiation enhances the invasive potential of primary glioblastoma cells via activation of the Rho signaling pathway. J Neurooncol. 2006;76(3):227–237. doi: 10.1007/s11060-005-6499-4. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

