Early versus deferred antiretroviral therapy for children older than 1 year infected with HIV (PREDICT): a multicentre, randomised, open-label trial
- PMID: 23059199
- PMCID: PMC3541427
- DOI: 10.1016/S1473-3099(12)70242-6
Early versus deferred antiretroviral therapy for children older than 1 year infected with HIV (PREDICT): a multicentre, randomised, open-label trial
Abstract
Background: The optimum time to start antiretroviral therapy for children diagnosed with HIV infection after 1 year of age is unknown. We assessed whether antiretroviral therapy could be deferred until CD4 percentages declined to less than 15% without affecting AIDS-free survival.
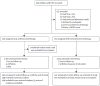
Methods: In our multicentre, randomised, open-label trial at nine research sites in Thailand and Cambodia, we enrolled children aged 1-12 years who were infected with HIV and had CD4 percentages of 15-24%. Participants were randomly assigned (1:1) by a minimisation scheme to start antiretroviral therapy at study entry (early treatment group) or antiretroviral therapy to start when CD4 percentages declined to less than 15% (deferred treatment group). The primary endpoint was AIDS-free survival (based on US Centers for Disease Control and Prevention category C events) at week 144, assessed with the Kaplan-Meier analysis and the log-rank approach. This study is registered with ClinicalTrials.gov, number NCT00234091.
Findings: Between March 28, 2006, and Sept 10, 2008, we enrolled 300 Thai and Cambodian children infected with HIV, with a median age of 6·4 years (IQR 3·9-8·4). 150 children were randomly allocated early antiretroviral therapy (one participant was excluded from analyses after withdrawing before week 0) and 150 children were randomly allocated deferred antiretroviral therapy. Median baseline CD4 percentage was 19% (16-22%). 69 children (46%) in the deferred treatment group started antiretroviral therapy during the study. AIDS-free survival at week 144 in the deferred treatment group was 98·7% (95% CI 94·7-99·7; 148 of 150 patients) compared with 97·9% (93·7-99·3; 146 of 149 patients) in the early treatment group (p=0·6).
Interpretation: AIDS-free survival in both treatment groups was high. This low event rate meant that our study was underpowered to detect differences between treatment start times and thus additional follow-up of study participants or future studies are needed to answer this clinical question.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
JA has received consulting fees or speaker’s honoraria from ViiV Healthcare, Abbott, and Gilead. KR has received consulting fees or speaker’s honoraria from ViiV Healthcare and Abbott, and support through grants HR1161A from the Thai National Research University Project of the Commission for Higher Education and the Ratchadaphiseksomphot Endowment Fund, Thailand; the Professional Researcher Strengthen Grant from the National Science and Technology Development Agency, BIOTEC; and Senior researcher scholar from the Thai Research Fund. All other authors declare that they have no conflicts of interest.
Figures


Comment in
-
When should therapy begin for children infected with HIV?Lancet Infect Dis. 2012 Dec;12(12):900-2. doi: 10.1016/S1473-3099(12)70266-9. Epub 2012 Oct 9. Lancet Infect Dis. 2012. PMID: 23059200 No abstract available.
References
-
- UNAIDS. [accessed June 22, 2012];UNAIDS report on the global AIDS epidemic. 2010 http://www.unaids.org/globalreport/Global_report.htm.
-
- Blanche S, Newell ML, Mayaux MJ, et al. Morbidity and mortality in European children vertically infected by HIV-1. The French Pediatric HIV Infection Study Group and European Collaborative Study. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:442–50. - PubMed
-
- Chearskul S, Chotpitayasunondh T, Simonds RJ, et al. and the Bangkok Collaborative Perinatal HIV Transmission Study Group. Survival, disease manifestations, and early predictors of disease progression among children with perinatal human immunodeficiency virus infection in Thailand. Pediatrics. 2002;110:e25. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

