Fibroblast growth factor control of cartilage homeostasis
- PMID: 23060229
- PMCID: PMC3690116
- DOI: 10.1002/jcb.24418
Fibroblast growth factor control of cartilage homeostasis
Abstract
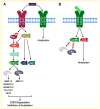
Osteoarthritis (OA) and degenerative disc disease (DDD) are similar diseases involving the breakdown of cartilage tissue, and a better understanding of the underlying biochemical processes involved in cartilage degeneration may allow for the development of novel biologic therapies aimed at slowing the disease process. Three members of the fibroblast growth factor (FGF) family, FGF-2, FGF-18, and FGF-8, have been implicated as contributing factors in cartilage homeostasis. The role of FGF-2 is controversial in both articular and intervertebral disc (IVD) cartilage as it has been associated with species- and age-dependent anabolic or catabolic events. Recent evidence suggests that FGF-2 selectively activates FGF receptor 1 (FGFR1) to exert catabolic effects in human articular chondrocytes and IVD tissue via upregulation of matrix-degrading enzyme production, inhibition of extracellular matrix (ECM) accumulation and proteoglycan synthesis, and clustering of cells characteristic of arthritic states. FGF-18, on the other hand, most likely exerts anabolic effects in human articular chondrocytes by activating the FGFR3 pathway, inducing ECM formation and chondrogenic cell differentiation, and inhibiting cell proliferation. These changes result in dispersed chondrocytes or disc cells surrounded by abundant matrix. The role of FGF-8 has recently been identified as a catabolic mediator in rat and rabbit articular cartilage, but its precise biological impact on human adult articular cartilage or IVD tissue remains unknown. The available evidence reveals the promise of FGF-2/FGFR1 antagonists, FGF-18/FGFR3 agonists, and FGF-8 antagonists (i.e., anti-FGF-8 antibody) as potential therapies to prevent cartilage degeneration and/or promote cartilage regeneration and repair in the future.
Copyright © 2012 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declared they have no conflict of interest.
Figures
Similar articles
-
Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis.Gene. 2008 Aug 15;420(1):82-9. doi: 10.1016/j.gene.2008.04.019. Epub 2008 May 9. Gene. 2008. PMID: 18565695 Free PMC article. Review.
-
Fibroblast growth factor receptor 1 is principally responsible for fibroblast growth factor 2-induced catabolic activities in human articular chondrocytes.Arthritis Res Ther. 2011 Aug 11;13(4):R130. doi: 10.1186/ar3441. Arthritis Res Ther. 2011. Retraction in: Arthritis Res Ther. 2024 Aug 7;26(1):149. doi: 10.1186/s13075-024-03381-y. PMID: 21835001 Free PMC article. Retracted.
-
Action of fibroblast growth factor-2 on the intervertebral disc.Arthritis Res Ther. 2008;10(2):R48. doi: 10.1186/ar2407. Epub 2008 Apr 24. Arthritis Res Ther. 2008. PMID: 18435858 Free PMC article.
-
Extracellular sulfatases support cartilage homeostasis by regulating BMP and FGF signaling pathways.Proc Natl Acad Sci U S A. 2010 Jun 1;107(22):10202-7. doi: 10.1073/pnas.0913897107. Epub 2010 May 17. Proc Natl Acad Sci U S A. 2010. PMID: 20479257 Free PMC article.
-
Regulation of FGF-2, FGF-18 and Transcription Factor Activity by Perlecan in the Maturational Development of Transitional Rudiment and Growth Plate Cartilages and in the Maintenance of Permanent Cartilage Homeostasis.Int J Mol Sci. 2022 Feb 9;23(4):1934. doi: 10.3390/ijms23041934. Int J Mol Sci. 2022. PMID: 35216048 Free PMC article. Review.
Cited by
-
Exercise-driven metabolic pathways in healthy cartilage.Osteoarthritis Cartilage. 2016 Jul;24(7):1210-22. doi: 10.1016/j.joca.2016.02.004. Epub 2016 Feb 27. Osteoarthritis Cartilage. 2016. PMID: 26924420 Free PMC article.
-
β-catenin as a key regulator of the cisplatin response in tumor cells.Clin Exp Med. 2025 Jun 15;25(1):206. doi: 10.1007/s10238-025-01757-1. Clin Exp Med. 2025. PMID: 40517336 Free PMC article. Review.
-
Inhibition of fibroblast growth factor receptor 3-dependent lung adenocarcinoma with a human monoclonal antibody.Dis Model Mech. 2016 May 1;9(5):563-71. doi: 10.1242/dmm.024760. Epub 2016 Apr 7. Dis Model Mech. 2016. PMID: 27056048 Free PMC article.
-
Role of sox9 in growth factor regulation of articular chondrocytes.J Cell Biochem. 2015 Jul;116(7):1391-400. doi: 10.1002/jcb.25099. J Cell Biochem. 2015. PMID: 25708223 Free PMC article.
-
Osteoblast role in the pathogenesis of rheumatoid arthritis.Mol Biol Rep. 2021 Mar;48(3):2843-2852. doi: 10.1007/s11033-021-06288-y. Epub 2021 Mar 27. Mol Biol Rep. 2021. PMID: 33774802 Free PMC article. Review.
References
-
- Alsalameh S, Mattka B, Al-Ward R, Lorenz HM, Manger B, Pfizenmaier K, Grell M, Kalden JR. Preferential expression of tumor necrosis factor receptor 55 (TNF-R55) on human articular chondrocytes: Selective transcriptional upregulation of TNF-R75 by proinflammatory cytokines interleukin 1beta, tumor necrosis factor-alpha, and basis fibroblast growth factor. J Rheumatol. 1999;26:645–653. - PubMed
-
- Blunt AG, Lawshe A, Cunningham ML, Seto ML, Ornitz DM, MacArthur CA. Overlapping expression and redundant activation of mesenchymal fibroblast growth factor (FGF) receptors by alternatively spliced FGF-8 ligands. J Biol Chem. 1997;272:3733–3738. - PubMed
-
- Chang Z, Meyer K, Rapraeger AC, Friedl A. Differential ability of heparan sulfate proteoglycans to assemble the fibroblast growth factor receptor complex in situ. FASEB J. 2000;14:137–144. - PubMed
-
- Chia SL, Sawaji Y, Burleigh A, McLean C, Inglis J, Saklatvala J, Vincent T. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum. 2009;60:2019–2027. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

