Structural studies of large nucleoprotein particles, vaults
- PMID: 23060231
- PMCID: PMC3491081
- DOI: 10.2183/pjab.88.416
Structural studies of large nucleoprotein particles, vaults
Abstract
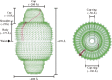
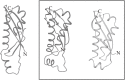
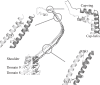
Vault is the largest nonicosahedral cytosolic nucleoprotein particle ever described. The widespread presence and evolutionary conservation of vaults suggest important biologic roles, although their functions have not been fully elucidated. X-ray structure of vault from rat liver was determined at 3.5 Å resolution. It exhibits an ovoid shape with a size of 40 × 40 × 67 nm(3). The cage structure of vault consists of a dimer of half-vaults, with each half-vault comprising 39 identical major vault protein (MVP) chains. Each MVP monomer folds into 12 domains: nine structural repeat domains, a shoulder domain, a cap-helix domain and a cap-ring domain. Interactions between the 42-turn-long cap-helix domains are key to stabilizing the particle. The other components of vaults, telomerase-associated proteins, poly(ADP-ribose) polymerases and small RNAs, are in location in the vault particle by electron microscopy.
Figures













References
-
- Mikyas Y., Makabi M., Raval-Fernandes S., Harrington L., Kickhoefer V.A., Rome L.H., Stewart P.L. (2004) Cryoelectron microscopy imaging of recombinant and tissue derived vaults: Localization of the MVP N termini and VPARP. J. Mol. Biol. 344, 91–105 - PubMed
-
- Tanaka H., Kato K., Yamashita E., Sumizawa T., Zhou Y., Yao M., Iwasaki K., Yoshimura M., Tsukihara T. (2009) The structure of rat liver vault at 3.5 angstrom resolution. Science 323, 384–388 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

