Voltage-dependent anion channels (VDACs) recruit Parkin to defective mitochondria to promote mitochondrial autophagy
- PMID: 23060438
- PMCID: PMC3504778
- DOI: 10.1074/jbc.M112.419721
Voltage-dependent anion channels (VDACs) recruit Parkin to defective mitochondria to promote mitochondrial autophagy
Abstract
Background: Parkin is recruited to defective mitochondria to promote degradation by an autophagy mechanism (mitophagy).
Results: VDACs specifically interact with Parkin on defective mitochondria and are required for efficient targeting of Parkin to mitochondria and subsequent mitophagy.
Conclusion: VDACs recruit Parkin to defective mitochondria.
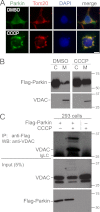
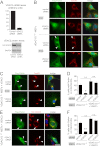
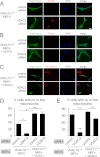
Significance: A novel mechanistic aspect of Parkin-dependent mitophagy is proposed that may be relevant to Parkinson disease. Mutations in the ubiquitin ligase Parkin and the serine/threonine kinase PINK1 can cause Parkinson disease. Both proteins function in the elimination of defective mitochondria by autophagy. In this process, activation of PINK1 mediates translocation of Parkin from the cytosol to mitochondria by an unknown mechanism. To better understand how Parkin is targeted to defective mitochondria, we purified affinity-tagged Parkin from mitochondria and identified Parkin-associated proteins by mass spectrometry. The three most abundant interacting proteins were the voltage-dependent anion channels 1, 2, and 3 (VDACs 1, 2, and 3), pore-forming proteins in the outer mitochondrial membrane. We demonstrate that Parkin specifically interacts with VDACs when the function of mitochondria is disrupted by treating cells with the proton uncoupler carbonyl cyanide p-chlorophenylhydrazone. In the absence of all three VDACs, the recruitment of Parkin to defective mitochondria and subsequent mitophagy are impaired. Each VDAC is sufficient to support Parkin recruitment and mitophagy, suggesting that VDACs can function redundantly. We hypothesize that VDACs serve as mitochondrial docking sites to recruit Parkin from the cytosol to defective mitochondria.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

