Efficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 study
- PMID: 23060624
- PMCID: PMC3485572
- DOI: 10.1183/09031936.00040712
Efficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 study
Abstract
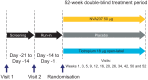
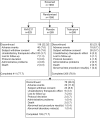
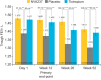
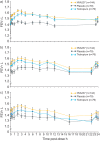
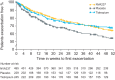
NVA237 (glycopyrronium bromide) is a once-daily long-acting muscarinic antagonist (LAMA) in development for chronic obstructive pulmonary disease (COPD). The GLycopyrronium bromide in COPD airWays clinical Study 2 (GLOW2) evaluated the efficacy and safety of NVA237 in moderate-to-severe COPD over 52 weeks. Patients were randomised 2:1:1 to NVA237 50 μg, placebo or open-label tiotropium 18 μg for 52 weeks. Primary end-point was trough forced expiratory volume in 1 s (FEV(1)) at 12 weeks. 1,066 patients were randomised, 810 completed the study. At week 12, trough FEV(1) increased significantly by 97 mL with NVA237 (95% CI 64.6-130.2; p<0.001) and 83 mL with tiotropium (95% CI 45.6-121.4; p<0.001). Compared with placebo, NVA237 produced significant improvements in dyspnoea (Transition Dyspnoea Index at week 26; p=0.002) and health status (St George's Respiratory Questionnaire at week 52; p<0.001). NVA237 significantly reduced the risk of moderate-to-severe COPD exacerbations by 34% (p=0.001) and the use of rescue medication (p=0.039), versus placebo. NVA237-placebo and tiotropium-placebo differences were comparable for all outcomes. Safety profiles were similar across groups. NVA237 50 μg provided significant improvements in lung function, dyspnoea, health status, exacerbations and rescue medication use, versus placebo, and was comparable to tiotropium. NVA237 can potentially be an alternative choice of LAMA for COPD patients.
Trial registration: ClinicalTrials.gov NCT00929110.
Conflict of interest statement
A statement of interest for all authors, and for the study itself, can be found at
Figures
References
-
- Viegi G, Pistelli F, Sherrill DL, et al. Definition, epidemiology and natural history of COPD. Eur Respir J 2007; 30: 993–1013 - PubMed
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD2011) Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Available from www.goldcopd.org. - PubMed
-
- Cooper N, Walker I, Knowles I. NVA237 has similar efficacy as tiotropium bromide against methacholine-induced bronchoconstriction and less systemic effect on cardiovascular variables in an anaesthetized rabbit model. Eur Respir J 2006; 28: Suppl. 50, 436s
-
- Trifilieff A, Cope N, Boivin JF, et al. The inhaled antimuscarinic receptor antagonist, NVA237 (glycopyrrolate), has a favorable side-effect profile in a Brown Norway rat lung function model when compared with tiotropium. Chest 2007; 132: 530a
-
- Fogarty C, Hattersley H, Di Scala L, et al. Bronchodilatory effects of NVA237, a once-daily long-acting muscarinic antagonist, in COPD patients. Respir Med 2011; 105;3: 337–342 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
